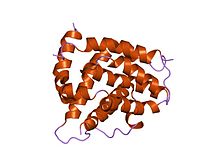
Bcl-2, encoded in humans by the BCL2 gene, is the founding member of the Bcl-2 family of regulator proteins that regulate cell death (apoptosis), by either inhibiting (anti-apoptotic) or inducing (pro-apoptotic) apoptosis. It was the first apoptosis regulator identified in any organism.

Apoptosis regulator BAX, also known as bcl-2-like protein 4, is a protein that in humans is encoded by the BAX gene. BAX is a member of the Bcl-2 gene family. BCL2 family members form hetero- or homodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. This protein forms a heterodimer with BCL2, and functions as an apoptotic activator. This protein is reported to interact with, and increase the opening of, the mitochondrial voltage-dependent anion channel (VDAC), which leads to the loss in membrane potential and the release of cytochrome c. The expression of this gene is regulated by the tumor suppressor P53 and has been shown to be involved in P53-mediated apoptosis.

The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains, and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.

The p53 upregulated modulator of apoptosis (PUMA) also known as Bcl-2-binding component 3 (BBC3), is a pro-apoptotic protein, member of the Bcl-2 protein family. In humans, the Bcl-2-binding component 3 protein is encoded by the BBC3 gene. The expression of PUMA is regulated by the tumor suppressor p53. PUMA is involved in p53-dependent and -independent apoptosis induced by a variety of signals, and is regulated by transcription factors, not by post-translational modifications. After activation, PUMA interacts with antiapoptotic Bcl-2 family members, thus freeing Bax and/or Bak which are then able to signal apoptosis to the mitochondria. Following mitochondrial dysfunction, the caspase cascade is activated ultimately leading to cell death.

Phorbol-12-myristate-13-acetate-induced protein 1 is a protein that in humans is encoded by the PMAIP1 gene, and is also known as Noxa.

Bcl-2 homologous antagonist/killer is a protein that in humans is encoded by the BAK1 gene on chromosome 6. The protein encoded by this gene belongs to the BCL2 protein family. BCL2 family members form oligomers or heterodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. This protein localizes to mitochondria, and functions to induce apoptosis. It interacts with and accelerates the opening of the mitochondrial voltage-dependent anion channel, which leads to a loss in membrane potential and the release of cytochrome c. This protein also interacts with the tumor suppressor P53 after exposure to cell stress.

B-cell lymphoma-extra large (Bcl-xL), encoded by the BCL2-like 1 gene, is a transmembrane molecule in the mitochondria. It is a member of the Bcl-2 family of proteins, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and ultimately, programmed cell death.
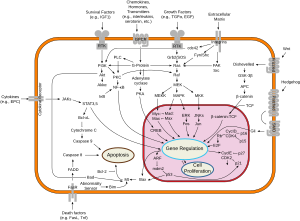
Inhibitors of apoptosis are a group of proteins that mainly act on the intrinsic pathway that block programmed cell death, which can frequently lead to cancer or other effects for the cell if mutated or improperly regulated. Many of these inhibitors act to block caspases, a family of cysteine proteases that play an integral role in apoptosis. Some of these inhibitors include the Bcl-2 family, viral inhibitor crmA, and IAP's.

Bcl-2-like protein 1 is a protein encoded in humans by the BCL2L1 gene. Through alternative splicing, the gene encodes both of the human proteins Bcl-xL and Bcl-xS.

Bcl-2-like protein 11, commonly called BIM, is a protein that in humans is encoded by the BCL2L11 gene.

14-3-3 protein theta is a protein that in humans is encoded by the YWHAQ gene.

Bcl-2-related protein A1 is a protein in humans which is encoded by the BCL2A1 gene.

Bcl-2-interacting killer is a protein that in humans is encoded by the BIK gene.

Bcl-2-like protein 2 is a 193-amino acid protein that in humans is encoded by the BCL2L2 gene on chromosome 14. It was originally discovered by Leonie Gibson, Suzanne Cory and colleagues at the Walter and Eliza Hall Institute of Medical Research, who called it Bcl-w.

Activator of apoptosis harakiri is a protein that in humans is encoded by the HRK gene.

Bcl-2-modifying factor is a protein that in humans is encoded by the BMF gene.

The mitochondrial apoptosis-induced channel, is an early marker of the onset of apoptosis. This ion channel is formed on the outer mitochondrial membrane in response to certain apoptotic stimuli. MAC activity is detected by patch clamping mitochondria from apoptotic cells at the time of cytochrome c release.

Bok is a protein-coding gene of the Bcl-2 family that is found in many invertebrates and vertebrates. It induces apoptosis, a special type of cell death. Currently, the precise function of Bok in this process is unknown.

Bcl-2-like protein 10 is a protein that in humans is encoded by the BCL2L10 gene.

The Bcl-2 family consists of a number of evolutionarily-conserved proteins that share Bcl-2 homology (BH) domains. The Bcl-2 family is most notable for their regulation of apoptosis, a form of programmed cell death, at the mitochondrion. The Bcl-2 family proteins consists of members that either promote or inhibit apoptosis, and control apoptosis by governing mitochondrial outer membrane permeabilization (MOMP), which is a key step in the intrinsic pathway of apoptosis. A total of 25 genes in the Bcl-2 family were identified by 2008.