T cells are one of the important types of white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface.

A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens (such as viruses or bacteria), or cells that are damaged in other ways.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

Natural killer cells, also known as NK cells, are a type of cytotoxic lymphocyte critical to the innate immune system. They are a kind of large granular lymphocytes (LGL), and belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.

Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B cells and secrete large quantities of proteins called antibodies in response to being presented specific substances called antigens. These antibodies are transported from the plasma cells by the blood plasma and the lymphatic system to the site of the target antigen, where they initiate its neutralization or destruction. B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell.
Alloimmunity is an immune response to nonself antigens from members of the same species, which are called alloantigens or isoantigens. Two major types of alloantigens are blood group antigens and histocompatibility antigens. In alloimmunity, the body creates antibodies against the alloantigens, attacking transfused blood, allotransplanted tissue, and even the fetus in some cases. Alloimmune (isoimmune) response results in graft rejection, which is manifested as deterioration or complete loss of graft function. In contrast, autoimmunity is an immune response to the self's own antigens. Alloimmunization (isoimmunization) is the process of becoming alloimmune, that is, developing the relevant antibodies for the first time.

An antigen-presenting cell (APC) or accessory cell is a cell that displays an antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T cells.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as helper T cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
CD38 (cluster of differentiation 38), also known as cyclic ADP ribose hydrolase, is a glycoprotein found on the surface of many immune cells (white blood cells), including CD4+, CD8+, B lymphocytes and natural killer cells. CD38 also functions in cell adhesion, signal transduction and calcium signaling.

CD3 is a protein complex and T cell co-receptor that is involved in activating both the cytotoxic T cell and T helper cells. It is composed of four distinct chains. In mammals, the complex contains a CD3γ chain, a CD3δ chain, and two CD3ε chains. These chains associate with the T-cell receptor (TCR) and the CD3-zeta (ζ-chain) to generate an activation signal in T lymphocytes. The TCR, CD3-zeta, and the other CD3 molecules together constitute the TCR complex.
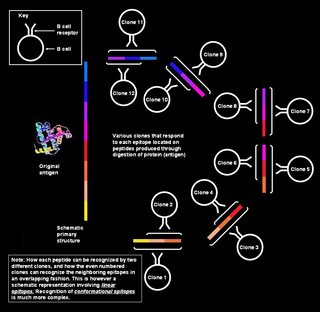
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.

CD48 antigen also known as B-lymphocyte activation marker (BLAST-1) or signaling lymphocytic activation molecule 2 (SLAMF2) is a protein that in humans is encoded by the CD48 gene.

Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the LAG3 gene. LAG3, which was discovered in 1990 and was designated CD223 after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biological effects on T cell function but overall has an immune inhibitory effect. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.

CD160 antigen is a protein that in humans is encoded by the CD160 gene.

T-cell surface glycoprotein CD8 alpha chain, is a protein encoded by CD8A gene.

White blood cells, also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. White blood cells are generally larger than red blood cells. They include three main subtypes: granulocytes, lymphocytes and monocytes.

Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.

Sialic acid-binding Ig-like lectin 6 is a protein that in humans is encoded by the SIGLEC6 gene. The gene was originally named CD33L (CD33-like) due to similarities between these genes but later became known as OB-BP1 due to its ability to bind to this factor and, finally, SIGLEC6 as the sixth member of the SIGLEC family of receptors to be identified. The protein has also been given the CD designation CD327.
















