
The epidermal growth factor receptor is a transmembrane protein that is a receptor for members of the epidermal growth factor family of extracellular protein ligands.

The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene on chromosome 11. CD44 has been referred to as HCAM, Pgp-1, Hermes antigen, lymphocyte homing receptor, ECM-III, and HUTCH-1.

Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.

Hyaluronan synthases (HAS) are membrane-bound enzymes that use UDP-α-N-acetyl-D-glucosamine and UDP-α-D-glucuronate as substrates to produce the glycosaminoglycan hyaluronan at the cell surface and extrude it through the membrane into the extracellular space.

Cyclin D1 is a protein that in humans is encoded by the CCND1 gene.
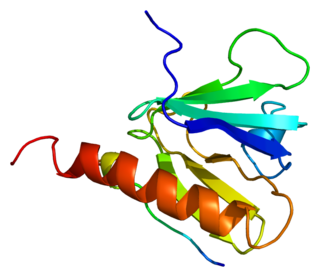
Insulin receptor substrate 1(IRS-1) is a signaling adapter protein that in humans is encoded by the IRS1 gene. It is a 131 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-terminus and a PTB domain ca. 40 residues downstream of this, followed by a poorly conserved C-terminus tail. Together with IRS2, IRS3 (pseudogene) and IRS4, it is homologous to the Drosophila protein chico, whose disruption extends the median lifespan of flies up to 48%. Similarly, Irs1 mutant mice experience moderate life extension and delayed age-related pathologies.

Serine/threonine-protein kinase PAK 1 is an enzyme that in humans is encoded by the PAK1 gene.

Rac GTPase-activating protein 1 is an enzyme that in humans is encoded by the RACGAP1 gene.

Mitotic checkpoint serine/threonine-protein kinase BUB1 beta is an enzyme that in humans is encoded by the BUB1B gene. Also known as BubR1, this protein is recognized for its mitotic roles in the spindle assembly checkpoint (SAC) and kinetochore-microtubule interactions that facilitate chromosome migration and alignment. BubR1 promotes mitotic fidelity and protects against aneuploidy by ensuring proper chromosome segregation between daughter cells. BubR1 is proposed to prevent tumorigenesis.

RhoC is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RHOC.

Neural precursor cell expressed developmentally down-regulated protein 9 (NEDD-9) is a protein that in humans is encoded by the NEDD9 gene. NEDD-9 is also known as enhancer of filamentation 1 (EF1), CRK-associated substrate-related protein (CAS-L), and Cas scaffolding protein family member 2 (CASS2). An important paralog of this gene is BCAR1.

Serine/threonine-protein kinase Nek2 is an enzyme that in humans is encoded by the NEK2 gene.

Hyaluronidase-2 is a multifunctional protein, previously thought to only possess acid-active hyaluronan-degrading enzymatic function. In humans it is encoded by the HYAL2 gene.

Targeting protein for Xklp2 is a protein that in humans is encoded by the TPX2 gene. It is one of the many spindle assembly factors that play a key role in inducing microtubule assembly and growth during M phase.

Hyaluronan synthase 2 is an enzyme that in humans is encoded by the HAS2 gene.

Large tumor suppressor kinase 1 (LATS1) is an enzyme that in humans is encoded by the LATS1 gene.

Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), also known as extracellular link domain containing 1 (XLKD1) is a Link domain-containing hyaladherin, a protein capable of binding to hyaluronic acid (HA), homologous to CD44, the main HA receptor. In humans it is encoded by the LYVE1 gene.

Aurora kinase C, also Serine/threonine-protein kinase 13 is an enzyme that in humans is encoded by the AURKC gene.

Serine/threonine-protein kinase Nek3 is an enzyme that in humans is encoded by the NEK3 gene.
Breast cancer is the most prevalent type of cancer among women globally, with 685,000 deaths recorded worldwide in 2020. The most commonly used treatment methods for breast cancer include surgery, radiotherapy and chemotherapy. Some of these treated patients experience disease relapse and metastasis. The aggressive progression and recurrence of this disease has been attributed the presence of a subset of tumor cells known as breast cancer stem cells (BCSCs). These cells possess the abilities of self-renewal and tumor initiation, allowing them to be drivers of metastases and tumor growth. The microenvironment in which these cells reside is filled with residential inflammatory cells that provide the needed signaling cues for BCSC-mediated self-renewal and survival. The production of cytokines allows these cells to escape from the primary tumor and travel through the circulation to distant organs, commencing the process of metastasis. Due to their significant role in driving disease progression, BCSCs represent a new target by which to treat the tumor at the source of metastasis.























