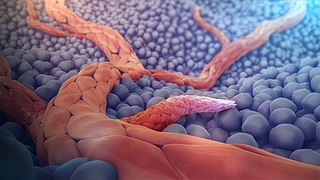
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

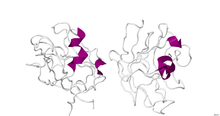
Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).

Pericytes are multi-functional mural cells of the microcirculation that wrap around the endothelial cells that line the capillaries throughout the body. Pericytes are embedded in the basement membrane of blood capillaries, where they communicate with endothelial cells by means of both direct physical contact and paracrine signaling. The morphology, distribution, density and molecular fingerprints of pericytes vary between organs and vascular beds. Pericytes help to maintain homeostatic and hemostatic functions in the brain, one of the organs with higher pericyte coverage, and also sustain the blood–brain barrier. These cells are also a key component of the neurovascular unit, which includes endothelial cells, astrocytes, and neurons. Pericytes have been postulated to regulate capillary blood flow and the clearance and phagocytosis of cellular debris in vitro. Pericytes stabilize and monitor the maturation of endothelial cells by means of direct communication between the cell membrane as well as through paracrine signaling. A deficiency of pericytes in the central nervous system can cause increased permeability of the blood–brain barrier.
Vascular endothelial growth factor, originally known as vascular permeability factor (VPF), is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis and angiogenesis.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

Angiopoietin is part of a family of vascular growth factors that play a role in embryonic and postnatal angiogenesis. Angiopoietin signaling most directly corresponds with angiogenesis, the process by which new arteries and veins form from preexisting blood vessels. Angiogenesis proceeds through sprouting, endothelial cell migration, proliferation, and vessel destabilization and stabilization. They are responsible for assembling and disassembling the endothelial lining of blood vessels. Angiopoietin cytokines are involved with controlling microvascular permeability, vasodilation, and vasoconstriction by signaling smooth muscle cells surrounding vessels. There are now four identified angiopoietins: ANGPT1, ANGPT2, ANGPTL3, ANGPT4.

VEGF receptors (VEGFRs) are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Depending on alternative splicing, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR).

Vascular endothelial growth factor receptor 1 is a protein that in humans is encoded by the FLT1 gene.

Platelet-derived growth factor receptor beta is a protein that in humans is encoded by the PDGFRB gene. Mutations in PDGFRB are mainly associated with the clonal eosinophilia class of malignancies.

Kinase insert domain receptor also known as vascular endothelial growth factor receptor 2 (VEGFR-2) is a VEGF receptor. KDR is the human gene encoding it. KDR has also been designated as CD309. KDR is also known as Flk1.

Low density lipoprotein receptor-related protein 1 (LRP1), also known as alpha-2-macroglobulin receptor (A2MR), apolipoprotein E receptor (APOER) or cluster of differentiation 91 (CD91), is a protein forming a receptor found in the plasma membrane of cells involved in receptor-mediated endocytosis. In humans, the LRP1 protein is encoded by the LRP1 gene. LRP1 is also a key signalling protein and, thus, involved in various biological processes, such as lipoprotein metabolism and cell motility, and diseases, such as neurodegenerative diseases, atherosclerosis, and cancer.

Neuropilin 2 (NRP2) is a protein that in humans is encoded by the NRP2 gene.
Vascular endothelial growth factor C (VEGF-C) is a protein that is a member of the platelet-derived growth factor / vascular endothelial growth factor (PDGF/VEGF) family. It is encoded in humans by the VEGFC gene, which is located on chromosome 4q34.

Neuropilin-1 is a protein that in humans is encoded by the NRP1 gene. In humans, the neuropilin 1 gene is located at 10p11.22. This is one of two human neuropilins.

Placental growth factor(PlGF) is a protein that in humans is encoded by the PGF gene.

Semaphorin-3A is a protein that in humans is encoded by the SEMA3A gene.

Platelet-derived growth factor C, also known as PDGF-C, is a 345-amino acid protein that in humans is encoded by the PDGFC gene. Platelet-derived growth factors are important in connective tissue growth, survival and function, and consist of disulphide-linked dimers involving two polypeptide chains, PDGF-A and PDGF-B. PDGF-C is a member of the PDGF/VEGF family of growth factors with a unique two-domain structure and expression pattern. PDGF-C was not previously identified with PDGF-A and PDGF-B, possibly because it may be that it is synthesized and secreted as a latent growth factor, requiring proteolytic removal of the N-terminal CUB domain for receptor binding and activation.
Vascular endothelial growth factor B also known as VEGF-B is a protein that, in humans, is encoded by the VEGF-B gene. VEGF-B is a growth factor that belongs to the vascular endothelial growth factor family, of which VEGF-A is the best-known member.

Vascular endothelial growth factor A (VEGF-A) is a protein that in humans is encoded by the VEGFA gene.
Neuroangiogenesis is the coordinated growth of nerves and blood vessels. The nervous and blood vessel systems share guidance cues and cell-surface receptors allowing for this synchronised growth. The term neuroangiogenesis only came into use in 2002 and the process was previously known as neurovascular patterning. The combination of neurogenesis and angiogenesis is an essential part of embryonic development and early life. It is thought to have a role in pathologies such as endometriosis, brain tumors, and Alzheimer's disease.