Related Research Articles

A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be easily distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface.

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules; however, these antibodies are not secreted. Rather, they are inserted into the plasma membrane where they serve as a part of B-cell receptors. When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell. Additionally, B cells present antigens and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, which is why the 'B' stands for bursa and not bone marrow as commonly believed.

A cytotoxic T cell is a T lymphocyte that kills cancer cells, cells that are infected, or cells that are damaged in other ways.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the immune system, particularly in the adaptive immune system. As their name suggests, they "help" the activity of other immune cells by releasing cytokines, small protein mediators that alter the behavior of target cells that express receptors for those cytokines. These cells help to polarize the immune response into the appropriate kind depending on the nature of the immunological insult (virus vs. extracellular bacterium vs. intracellular bacterium vs. helminth vs. fungus vs. protist). They are generally considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.

Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system that belong to the rapidly expanding family of innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, acting at around 3 days after infection, and respond to tumor formation. Typically, immune cells detect the major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release, causing the death of the infected cell by lysis or apoptosis. NK cells are unique, however, as they have the ability to recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class 1. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.

Plasma cells, also called plasma B cells, are white blood cells that originate in the bone marrow and secrete large quantities of proteins called antibodies in response to being presented specific substances called antigens. These antibodies are transported from the plasma cells by the blood plasma and the lymphatic system to the site of the target antigen, where they initiate its neutralization or destruction. B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell.
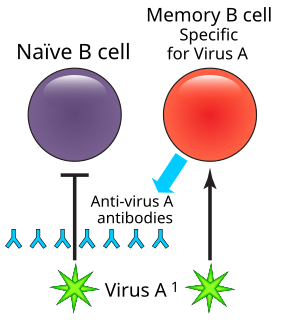
In immunology, a memory B cell (MBC) is a type of B lymphocyte that forms part of the adaptive immune system. These cells develop within germinal centers of the secondary lymphoid organs. Memory B cells circulate in the blood stream in a quiescent state, sometimes for decades. Their function is to memorize the characteristics of the antigen that activated their parent B cell during initial infection such that if the memory B cell later encounters the same antigen, it triggers an accelerated and robust secondary immune response. Memory B cells have B cell receptors (BCRs) on their cell membrane, identical to the one on their parent cell, that allow them to recognize antigen and mount a specific antibody response.

An antigen-presenting cell (APC) or accessory cell is a cell that displays antigen complexed with major histocompatibility complexes (MHCs) on their surfaces; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T-cells.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
A Thymocyte is an immune cell present in the thymus, before it undergoes transformation into a T cell. Thymocytes are produced as stem cells in the bone marrow and reach the thymus via the blood. Thymopoiesis describes the process which turns thymocytes into mature T cells according to either negative or positive selection. This selection process is vitally important in shaping the population of thymocytes into a peripheral pool of T cells that are able to respond to foreign pathogens but remain tolerant towards the body's own antigens. Positive selection selects cells which are able to bind MHC class I or II molecules with at least a weak affinity. This eliminates those T cells which would be non-functional due to an inability to bind MHC. Negative selection destroys thymocytes with a high affinity for self peptides or MHC. This eliminates cells which would direct immune responses towards self-proteins in the periphery. Negative selection is not 100% effective, and some autoreactive T cells escape and are released into the circulation. Additional mechanisms of peripheral tolerance exist to silence these cells, but if these fail, autoimmunity may arise.

Antigen presentation is a vital immune process that is essential for T cell immune response triggering. Because T cells recognize only fragmented antigens displayed on cell surfaces, antigen processing must occur before the antigen fragment, now bound to the major histocompatibility complex (MHC), is transported to the surface of the cell, a process known as presentation, where it can be recognized by a T-cell receptor. If there has been an infection with viruses or bacteria, the cell will present an endogenous or exogenous peptide fragment derived from the antigen bound to MHC molecules. There are two types of MHC molecules which differ in the behaviour of the antigens: MHC class I molecules (MHC-I) bind peptides from the cell cytosol, while peptides generated in the endocytic vesicles after internalisation are bound to MHC class II (MHC-II). Cellular membranes separate these two cellular environments - intracellular and extracellular. Each T cell can only recognize tens to hundreds of copies of a unique sequence of a single peptide among thousands of other peptides presented on the same cell, because an MHC molecule in one cell can bind to quite a large range of peptides.

MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on professional antigen-presenting cells such as dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial cells, and B cells. These cells are important in initiating immune responses.

B-lymphocyte antigen CD20 or CD20 is expressed on the surface of all B-cells beginning at the pro-B phase and progressively increasing in concentration until maturity.
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) is the generation of lymphocytes, one of the five types of white blood cell (WBC). It is more formally known as lymphoid hematopoiesis.

B-lymphocyte antigen CD19, also known as CD19 molecule, B-Lymphocyte Surface Antigen B4, T-Cell Surface Antigen Leu-12 and CVID3 is a transmembrane protein that in humans is encoded by the gene CD19. In humans, CD19 is expressed in all B lineage cells. Contrary to some early doubts, human plasma cells do express CD19, as confirmed by others. CD19 plays two major roles in human B cells: on the one hand, it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane; on the other, it works within the CD19/CD21 complex to decrease the threshold for B cell receptor signaling pathways. Due to its presence on all B cells, it is a biomarker for B lymphocyte development, lymphoma diagnosis and can be utilized as a target for leukemia immunotherapies.
Understanding of the antitumor immunity role of CD4+ T cells has grown substantially since the late 1990s. CD4+ T cells play an important role in modulating immune responses to pathogens and tumor cells, and are important in orchestrating overall immune responses.

HLA-DM is an intracellular protein involved in the mechanism of antigen presentation on antigen presenting cells (APCs) of the immune system. It does this by assisting in peptide loading of major histocompatibility complex (MHC) class II membrane-bound proteins. HLA-DM is encoded by the genes HLA-DMA and HLA-DMB.

Lymphocyte-activation gene 3, also known as LAG-3, is a protein which in humans is encoded by the LAG3 gene. LAG3, which was discovered in 1990 and was designated CD223 after the Seventh Human Leucocyte Differentiation Antigen Workshop in 2000, is a cell surface molecule with diverse biologic effects on T cell function. It is an immune checkpoint receptor and as such is the target of various drug development programs by pharmaceutical companies seeking to develop new treatments for cancer and autoimmune disorders. In soluble form it is also being developed as a cancer drug in its own right.
Marginal zone B cells are noncirculating mature B cells that in humans segregate anatomically into the marginal zone (MZ) of the spleen and certain other types of lymphoid tissue. The MZ B cells within this region typically express low-affinity polyreactive B-cell receptors (BCR), high levels of IgM, Toll-like receptors (TLRs), CD21, CD1, CD9, CD27 with low to negligible levels of secreted-IgD, CD23, CD5, and CD11b that help to distinguish them phenotypically from follicular (FO) B cells and B1 B cells.
References
- 1 2 Slack JL, Armitage RJ, Ziegler SF, Dower SK, Gruss HJ (July 1995). "Molecular characterization of the pan-B cell antigen CDw78 as a MHC class II molecule by direct expression cloning of the transcription factor CIITA". International Immunology. 7 (7): 1087–92. doi:10.1093/intimm/7.7.1087. PMID 8527406.
- 1 2 3 "CD78 antibody (60-3G2) (ab24151) datasheet". Abcam. Retrieved 2008-10-28.
- 1 2 Bona, Constantin; Francisco A. Bonilla (1996). "5". Textbook of Immunology. Martin Soohoo (2 ed.). CRC Press. p. 102. ISBN 978-3-7186-0596-5.