An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes.
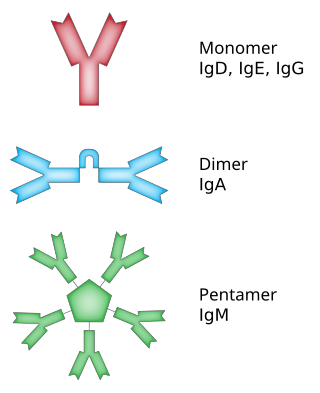
Immunoglobulin D (IgD) is an antibody isotype that makes up about 1% of proteins in the plasma membranes of immature B-lymphocytes where it is usually co-expressed with another cell surface antibody called IgM. IgD is also produced in a secreted form that is found in very small amounts in blood serum, representing 0.25% of immunoglobulins in serum. The relative molecular mass and half-life of secreted IgD is 185 kDa and 2.8 days, respectively. Secreted IgD is produced as a monomeric antibody with two heavy chains of the delta (δ) class, and two Ig light chains.
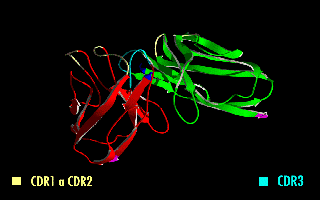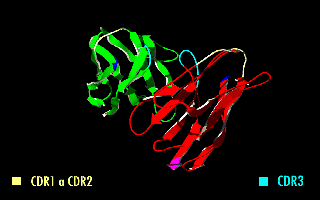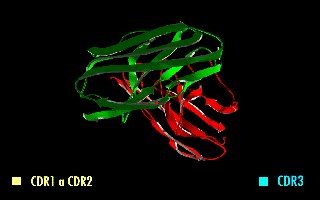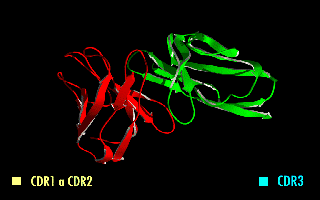
A single-chain variable fragment (scFv) is not actually a fragment of an antibody, but instead is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or vice versa. This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker. The image to the right shows how this modification usually leaves the specificity unaltered.
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked N-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched.

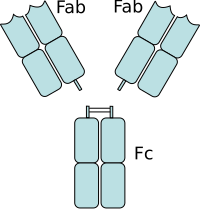
In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Protein A is a 42 kDa surface protein originally found in the cell wall of the bacteria Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation, the bacteria disrupts opsonization and phagocytosis.
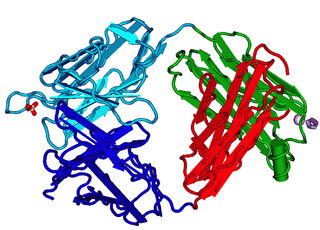
The fragment antigen-binding region is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope, comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen.
CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.

In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV). The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding. The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells. While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen. Naturally occurring antibodies typically only target one antigen. BsAbs can be manufactured in several structural formats. BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes. BsAbs have been explored for cancer immunotherapy, drug delivery, and Alzeimer's disease.

Immunoglobulin heavy constant alpha 1 is a immunoglobulin gene with symbol IGHA1. It encodes a constant (C) segment of Immunoglobulin A heavy chain. Immunoglobulin A is an antibody that plays a critical role in immune function in the mucous membranes. IgA shows the same typical structure of other antibody classes, with two heavy chains and two light chains, and four distinct domains: one variable region, and three variable regions. As a major class of immunoglobulin in body secretions, IgA plays a role in defending against infection, as well as preventing the access of foreign antigens to the immunologic system.
The following outline is provided as an overview of and topical guide to immunology:
Primary and secondary antibodies are two groups of antibodies that are classified based on whether they bind to antigens or proteins directly or target another (primary) antibody that, in turn, is bound to an antigen or protein.

Two chemically linked fragments antigen-binding form an artificial antibody that binds to two different antigens, making it a type of bispecific antibody. They are fragments antigen-binding of two different monoclonal antibodies and are linked by chemical means like a thioether. Typically, one of the Fabs binds to a tumour antigen and the other to a protein on the surface of an immune cell, for example an Fc receptor on a macrophage. In this way, tumour cells are attached to immune cells, which destroy them.
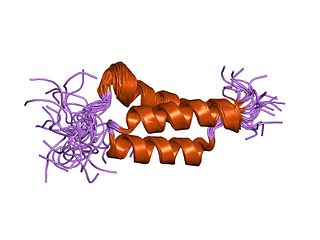
In molecular biology, the domain B, refers to the immunoglobulin-binding domain found in the Staphylococcus aureus virulence factor protein A (SpA). Hence, it is abbreviated to SpAB.
Fcabs are antibodies fragments engineered from the constant region of an antibody (Fc). In naturally occurring antibodies, the antigen-binding sites are located at the variable regions (Fab).
F-star Biotechnology Ltd. is a biotechnology company, founded in Vienna in 2006, with a current main research site in Cambridge, UK. The company is focused in developing bispecific monoclonal antibodies using a modular combinatorial approach that engineers the Fc constant region of an immunoglobulin into a novel antigen-binding site.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.