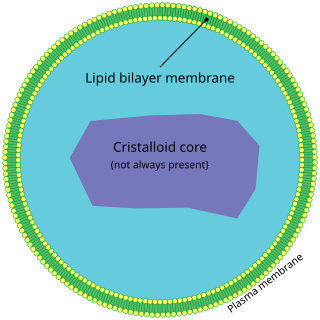
A peroxisome (IPA: [pɛɜˈɹɒksɪˌsoʊm]) is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen peroxide (H2O2) is then formed. Peroxisomes owe their name to hydrogen peroxide generating and scavenging activities. They perform key roles in lipid metabolism and the conversion of reactive oxygen species. Peroxisomes are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, bile acid intermediates (in the liver), D-amino acids, and polyamines, the reduction of reactive oxygen species – specifically hydrogen peroxide – and the biosynthesis of plasmalogens, i.e., ether phospholipids critical for the normal function of mammalian brains and lungs. They also contain approximately 10% of the total activity of two enzymes (Glucose-6-phosphate dehydrogenase and 6-Phosphogluconate dehydrogenase) in the pentose phosphate pathway, which is important for energy metabolism. It is vigorously debated whether peroxisomes are involved in isoprenoid and cholesterol synthesis in animals. Other known peroxisomal functions include the glyoxylate cycle in germinating seeds ("glyoxysomes"), photorespiration in leaves, glycolysis in trypanosomes ("glycosomes"), and methanol and/or amine oxidation and assimilation in some yeasts.

Zellweger syndrome is a rare congenital disorder characterized by the reduction or absence of functional peroxisomes in the cells of an individual. It is one of a family of disorders called Zellweger spectrum disorders which are leukodystrophies. Zellweger syndrome is named after Hans Zellweger (1909–1990), a Swiss-American pediatrician, a professor of pediatrics and genetics at the University of Iowa who researched this disorder.
Refsum disease is an autosomal recessive neurological disease that results in the over-accumulation of phytanic acid in cells and tissues. It is one of several disorders named after Norwegian neurologist Sigvald Bernhard Refsum (1907–1991). Refsum disease typically is adolescent onset and is diagnosed by above average levels of phytanic acid. Humans obtain the necessary phytanic acid primarily through diet. It is still unclear what function phytanic acid plays physiologically in humans, but has been found to regulate fatty acid metabolism in the liver of mice.

Peroxisomal disorders represent a class of medical conditions caused by defects in peroxisome functions. This may be due to defects in single enzymes important for peroxisome function or in peroxins, proteins encoded by PEX genes that are critical for normal peroxisome assembly and biogenesis.
Infantile Refsum disease (IRD) is a rare autosomal recessive congenital peroxisomal biogenesis disorder within the Zellweger spectrum. These are disorders of the peroxisomes that are clinically similar to Zellweger syndrome and associated with mutations in the PEX family of genes. IRD is associated with deficient phytanic acid catabolism, as is adult Refsum disease, but they are different disorders that should not be confused.

Peroxisomal targeting signal 1 receptor (PTS1R) is a protein that in humans is encoded by the PEX5 gene.

Peroxisome biogenesis factor 1, also known as PEX1, is a protein which in humans is encoded by the PEX1 gene.
Peroxisomal biogenesis factor 19 is a protein that in humans is encoded by the PEX19 gene.
Peroxisomal membrane protein PEX14 is a protein that in humans is encoded by the PEX14 gene.

ATP-binding cassette sub-family D member 3 is a protein that in humans is encoded by the ABCD3 gene.

Peroxisomal biogenesis factor 2 is a protein that in humans is encoded by the PEX2 gene.

Peroxisome assembly protein 12 is a protein that in humans is encoded by the PEX12 gene.

Peroxisomal membrane protein PEX13 is a protein that in humans is encoded by the PEX13 gene. It located on chromosome 2 next to KIAA1841

Peroxisomal biogenesis factor 3 is a protein that in humans is encoded by the PEX3 gene.

Peroxisome biogenesis factor 10 is a protein that in humans is encoded by the PEX10 gene. Alternative splicing results in two transcript variants encoding different isoforms.

Peroxisomal membrane protein PEX16 is a protein that in humans is encoded by the PEX16 gene.

Peroxisome assembly protein 26 is a protein that in humans is encoded by the PEX26 gene.

Peroxisomal membrane protein 11B is a protein that in humans is encoded by the PEX11B gene. It is involved in the regulation of peroxisome abundance.

Peroxisomal membrane protein 11A is a protein that in humans is encoded by the PEX11A gene.
Zellweger spectrum disorders are a group of rare disorders that create the same disease process. The subdivisions of this spectrum are hyperpipecolic acidemia, infantile Refsum disease, neonatal adrenoleukodystrophy, and Zellweger syndrome. It can also be referred to as peroxisomal biogenesis disorders, Zellweger syndrome spectrum, NALD, cerebrohepatorenal syndrome, and ZSS. It can affect many body organs, including the kidneys, eyes, and hearing. It is named after Hans Zellweger.