Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

A lysosome is a single membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that digest many kinds of biomolecules. A lysosome has a specific composition, of both its membrane proteins and its lumenal proteins. The lumen's pH (~4.5–5.0) is optimal for the enzymes involved in hydrolysis, analogous to the activity of the stomach. Besides degradation of polymers, the lysosome is involved in cell processes of secretion, plasma membrane repair, apoptosis, cell signaling, and energy metabolism.

In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell.
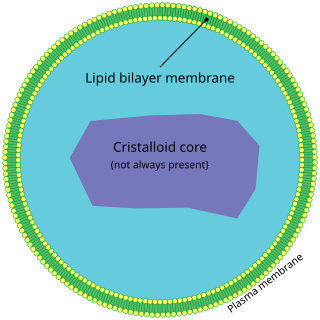
A peroxisome (IPA:[pɛɜˈɹɒksɪˌsoʊm]) is a membrane-bound organelle, a type of microbody, found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen peroxide (H2O2) is then formed. Peroxisomes owe their name to hydrogen peroxide generating and scavenging activities. They perform key roles in lipid metabolism and the reduction of reactive oxygen species.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Anabolism is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.
Digestion is the breakdown of carbohydrates to yield an energy-rich compound called ATP. The production of ATP is achieved through the oxidation of glucose molecules. In oxidation, the electrons are stripped from a glucose molecule to reduce NAD+ and FAD. NAD+ and FAD possess a high energy potential to drive the production of ATP in the electron transport chain. ATP production occurs in the mitochondria of the cell. There are two methods of producing ATP: aerobic and anaerobic. In aerobic respiration, oxygen is required. Using oxygen increases ATP production from 4 ATP molecules to about 30 ATP molecules. In anaerobic respiration, oxygen is not required. When oxygen is absent, the generation of ATP continues through fermentation. There are two types of fermentation: alcohol fermentation and lactic acid fermentation.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.
The term amphibolism is used to describe a biochemical pathway that involves both catabolism and anabolism. Catabolism is a degradative phase of metabolism in which large molecules are converted into smaller and simpler molecules, which involves two types of reactions. First, hydrolysis reactions, in which catabolism is the breaking apart of molecules into smaller molecules to release energy. Examples of catabolic reactions are digestion and cellular respiration, where sugars and fats are broken down for energy. Breaking down a protein into amino acids, or a triglyceride into fatty acids, or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions. Second, oxidation reactions involve the removal of hydrogens and electrons from an organic molecule. Anabolism is the biosynthesis phase of metabolism in which smaller simple precursors are converted to large and complex molecules of the cell. Anabolism has two classes of reactions. The first are dehydration synthesis reactions; these involve the joining of smaller molecules together to form larger, more complex molecules. These include the formation of carbohydrates, proteins, lipids and nucleic acids. The second are reduction reactions, in which hydrogens and electrons are added to a molecule. Whenever that is done, molecules gain energy.
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of thousands of different cellular processes such as cellular respiration and the many other metabolic and enzymatic processes that lead to production and utilization of energy in forms such as adenosine triphosphate (ATP) molecules. That is, the goal of bioenergetics is to describe how living organisms acquire and transform energy in order to perform biological work. The study of metabolic pathways is thus essential to bioenergetics.
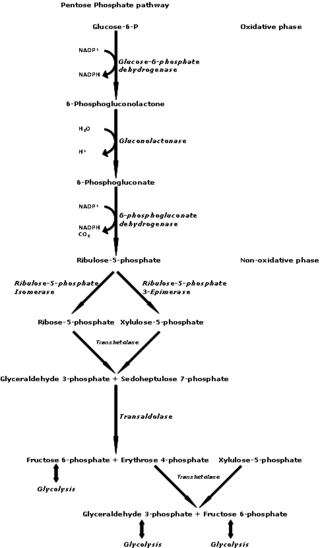
The pentose phosphate pathway is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides. While the pentose phosphate pathway does involve oxidation of glucose, its primary role is anabolic rather than catabolic. The pathway is especially important in red blood cells (erythrocytes). The reactions of the pathway were elucidated in the early 1950s by Bernard Horecker and co-workers.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.
Endoplasm generally refers to the inner, dense part of a cell's cytoplasm. This is opposed to the ectoplasm which is the outer (non-granulated) layer of the cytoplasm, which is typically watery and immediately adjacent to the plasma membrane. The nucleus is separated from the endoplasm by the nuclear envelope. The different makeups/viscosities of the endoplasm and ectoplasm contribute to the amoeba's locomotion through the formation of a pseudopod. However, other types of cells have cytoplasm divided into endo- and ectoplasm. The endoplasm, along with its granules, contains water, nucleic acids, amino acids, carbohydrates, inorganic ions, lipids, enzymes, and other molecular compounds. It is the site of most cellular processes as it houses the organelles that make up the endomembrane system, as well as those that stand alone. The endoplasm is necessary for most metabolic activities, including cell division.
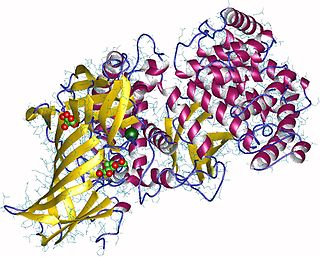
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the N-terminus (beginning), of proteins or peptides. They are found in many organisms; in the cell, they are found in many organelles, in the cytosol, and as membrane proteins. Aminopeptidases are used in essential cellular functions, and are often zinc metalloenzymes, containing a zinc cofactor.

The glycosome is a membrane-enclosed organelle that contains the glycolytic enzymes. The term was first used by Scott and Still in 1968 after they realized that the glycogen in the cell was not static but rather a dynamic molecule. It is found in a few species of protozoa including the Kinetoplastida which include the suborders Trypanosomatida and Bodonina, most notably in the human pathogenic trypanosomes, which can cause sleeping sickness, Chagas's disease, and leishmaniasis. The organelle is bounded by a single membrane and contains a dense proteinaceous matrix. It is believed to have evolved from the peroxisome. This has been verified by work done on Leishmania genetics.
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP). More generally, in the medical literature, the Pasteur effect refers to how the cellular presence of oxygen causes in cells a decrease in the rate of glycolysis and also a suppression of lactate accumulation. The effect occurs in animal tissues, as well as in microorganisms belonging to the fungal kingdom.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.
The following outline is provided as an overview of and topical guide to cell biology: