Dipeptidases are enzymes secreted by enterocytes into the small intestine. Dipeptidases hydrolyze bound pairs of amino acids, called dipeptides.

Enzymes are macromolecular biological catalysts that accelerate chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.

Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small intestine. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase its surface area. This facilitates transport of numerous small molecules into the enterocyte from the intestinal lumen. These include broken down proteins, fats, and sugars, as well as water, electrolytes, vitamins, and bile salts. Enterocytes also have a endocrine role, secreting hormones such as leptin.
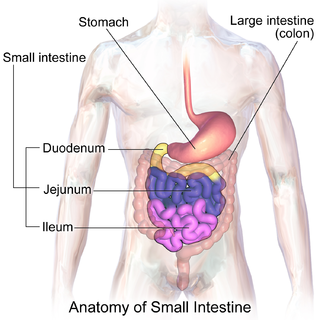
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the end absorption of nutrients and minerals from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion.
Dipeptidases are secreted onto the brush border of the villi in the small intestine, where they cleave dipeptides into their two component amino acids prior to absorption.
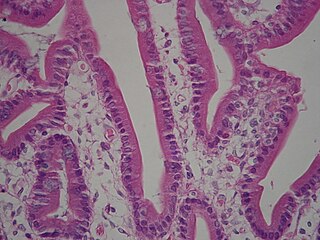
A brush border is the microvilli-covered surface of simple cuboidal epithelium and simple columnar epithelium cells found in certain locations of the body. Microvilli are approximately 100 nanometers in diameter and their length varies from approximately 100 to 2,000 nanometers in length. Because individual microvilli are so small and are tightly packed in the brush border, individual microvilli can only be resolved using electron microscopes; with a light microscope they can usually only be seen collectively as a fuzzy fringe at the surface of the epithelium. This fuzzy appearance gave rise to the term brush border, as early anatomists noted that this structure appeared very much like the bristles of a paintbrush.
DIPEPTIDASES convert dipeptides into amino acids
Dipeptidases are also found within the enterocytes themselves, performing cytosolic digestion of absorbed dipeptides.
They are exopeptidases, classified under EC number 3.4.13.
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal peptide bond; the process releases a single amino acid or dipeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal, an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide.
The Enzyme Commission number is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze. As a system of enzyme nomenclature, every EC number is associated with a recommended name for the respective enzyme.

The ileum is the final section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear and the terms posterior intestine or distal intestine may be used instead of ileum. Its main function is to absorb vitamin B12, bile salts, and whatever products of digestion were not absorbed by the jejunum.

The jejunum is the second part of the small intestine in humans and most higher vertebrates, including mammals, reptiles, and birds. Its lining is specialized for the absorption by enterocytes of small nutrient molecules which have been previously digested by enzymes in the duodenum.

Intestinal villi are small, finger-like projections that extend into the lumen of the small intestine. Each villus is approximately 0.5–1.6 mm in length, and has many microvilli projecting from the enterocytes of its epithelium which collectively form the striated or brush border. Each of these microvilli are much smaller than a single villus. The intestinal villi are much smaller than any of the circular folds in the intestine.

A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.

Chylomicrons are lipoprotein particles that consist of triglycerides (85–92%), phospholipids (6–12%), cholesterol (1–3%), and proteins (1–2%). Due to their density relative to lipoproteins, they are also commonly known as ultra low density lipoproteins (ULDL) in modern usage. They transport dietary lipids from the intestines to other locations in the body. ULDLs are one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. A protein specific to chylomicrons is ApoB48.
Gastric acid, gastric juice, or stomach acid, is a digestive fluid formed in the stomach and is composed of hydrochloric acid (HCl), potassium chloride (KCl), and sodium chloride (NaCl). The acid plays a key role in digestion of proteins, by activating digestive enzymes, and making ingested proteins unravel so that digestive enzymes break down the long chains of amino acids. Gastric acid is produced by cells in the lining of the stomach, which are coupled in feedback systems to increase acid production when needed. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring that it does not become too acidic. These cells also produce mucus, which forms a viscous physical barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to completely neutralize any gastric acid that passes further down into the digestive tract.
Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption by the body. Digestive enzymes are found in the digestive tracts of animals and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines.

Enterohepatic circulation refers to the circulation of biliary acids, bilirubin, drugs or other substances from the liver to the bile, followed by entry into the small intestine, absorption by the enterocyte and transport back to the liver. Enterohepatic circulation is an especially important concept in the field of toxicology as many lipophilic xenobiotics undergo this process causing repeated liver damage.
Erepsin is a mixture of enzymes contained in a protein fraction found in the intestinal juices that digest peptones into amino acids. It is produced and secreted by the intestinal glands in the ileum and the pancreas, but it is also found widely in other cells. It is however a term now rarely used in scientific literature as more precise terms are preferred.

In histology, an intestinal gland is a gland found in between villi in the intestinal epithelium lining of the small intestine and large intestine. The glands and intestinal villi are covered by epithelium, which contains multiple types of cells: enterocytes, goblet cells, enteroendocrine cells, cup cells, tuft cells, and at the base of the gland, Paneth cells and stem cells.
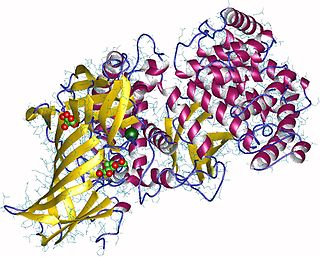
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides(exopeptidases).They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes.

Dipeptidase 1 (DPEP1), or renal dipeptidase, is a membrane-bound glycoprotein responsible for hydrolyzing dipeptides. It is found in the microsomal fraction of the procine kidney cortex. It exists as a disulfide-linked homodimer that is glygosylphosphatidylinositol (GPI)-anchored to the renal brush border of the kidney. The active site on each homodimer is made up of a barrel subunit with binuclear zinc ions that are bridged by the Gly125 side-chain located at the bottom of the barrel.
Xaa-methyl-His dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction

Xaa-Pro dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Non-stereospecific dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Cytosol nonspecific dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
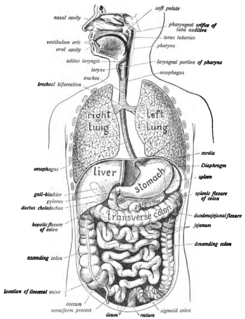
The human digestive system consists of the gastrointestinal tract plus the accessory organs of digestion. Digestion involves the breakdown of food into smaller and smaller components, until they can be absorbed and assimilated into the body. The process of digestion has three stages. The first stage is the cephalic phase of digestion which begins with gastric secretions in response to the sight and smell of food. This stage includes the mechanical breakdown of food by chewing, and the chemical breakdown by digestive enzymes, that takes place in the mouth.











