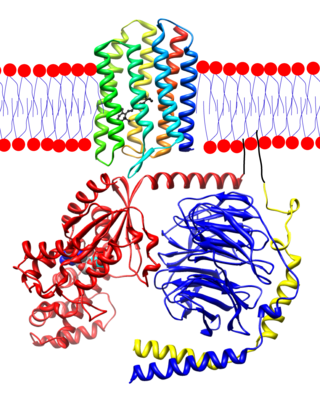
 Membrane-bound ADP ribosylation factor-like protein 2 (ARL2 mouse, red), complex with phosphodiesterase delta (yellow) ( 1ksg ) Blue dots show hydrocarbon boundary of the lipid bilayer | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Arf | ||||||||
| Pfam | PF00025 | ||||||||
| InterPro | IPR006689 | ||||||||
| SMART | ARF | ||||||||
| PROSITE | PDOC01020 | ||||||||
| SCOP2 | 1hur / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 124 | ||||||||
| OPM protein | 1ksg | ||||||||
| CDD | cd00878 | ||||||||
| Membranome | 1103 | ||||||||
| |||||||||

ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling.
Contents
- Regulatory proteins
- GTP/GDP exchange proteins
- Other proteins
- Phylogeny
- Structure
- Examples
- See also
- References
- Further reading
- External links
The small ADP ribosylation factor (Arf) GTP-binding proteins are major regulators of vesicle biogenesis in intracellular traffic. [1] They are the founding members of a growing family that includes Arl (Arf-like), Arp (Arf-related proteins) and the remotely related Sar (Secretion-associated and Ras-related) proteins. Arf proteins cycle between inactive GDP-bound and active GTP-bound forms that bind selectively to effectors. The classical structural GDP/GTP switch is characterised by conformational changes at the so-called switch 1 and switch 2 regions, which bind tightly to the gamma-phosphate of GTP but poorly or not at all to the GDP nucleotide. Structural studies of Arf1 and Arf6 have revealed that although these proteins feature the switch 1 and 2 conformational changes, they depart from other small GTP-binding proteins in that they use an additional, unique switch to propagate structural information from one side of the protein to the other.
The GDP/GTP structural cycles of human Arf1 and Arf6 feature a unique conformational change that affects the beta2beta3 strands connecting switch 1 and switch 2 (interswitch) and also the amphipathic helical N-terminus. In GDP-bound Arf1 and Arf6, the interswitch is retracted and forms a pocket to which the N-terminal helix binds, the latter serving as a molecular hasp to maintain the inactive conformation. In the GTP-bound form of these proteins, the interswitch undergoes a two-residue register shift that pulls switch 1 and switch 2 up, restoring an active conformation that can bind GTP. In this conformation, the interswitch projects out of the protein and extrudes the N-terminal hasp by occluding its binding pocket.