
The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
A cisterna is a flattened membrane vesicle found in the endoplasmic reticulum and Golgi apparatus. Cisternae are an integral part of the packaging and modification processes of proteins occurring in the Golgi.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

Brefeldin A is a lactone antiviral produced by the fungus Penicillium brefeldianum. Brefeldin A inhibits protein transport from the endoplasmic reticulum to the golgi complex indirectly by preventing association of COP-I coat to the Golgi membrane. Brefeldin A was initially isolated with hopes to become an antiviral drug but is now primarily used in research to study protein transport.

ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling.
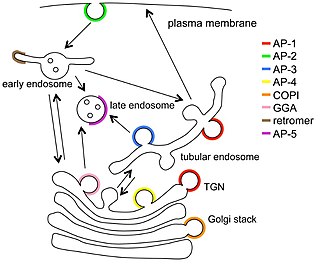
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

The endoplasmic-reticulum–Golgi intermediate compartment (ERGIC) is an organelle in eukaryotic cells. This compartment mediates transport between the endoplasmic reticulum (ER) and Golgi complex, facilitating the sorting of cargo. The cluster was first identified in 1988 using an antibody to the protein that has since been named ERGIC-53. It is also referred to as the vesicular-tubular cluster (VTC) or, originally, tubulo-vesicular compartment.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.

ADP-ribosylation factor 1 is a protein that in humans is encoded by the ARF1 gene.

Coatomer subunit beta is a protein that in humans is encoded by the COPB1 gene.

Coatomer subunit alpha is a protein that in humans is encoded by the COPA gene.

Coatomer subunit epsilon is a protein that in humans is encoded by the COPE gene.

Coatomer subunit beta is a protein that is encoded by the COPB2 gene in humans.

Coatomer subunit gamma is a protein that in humans is encoded by the COPG gene. It is one of seven proteins in the COPI coatomer complex that coats vesicles as they bud from the Golgi complex.
KDEL is a target peptide sequence in mammals and plants located on the C-terminal end of the amino acid structure of a protein. The KDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

The C-terminal domain ofBeta2-adaptin is a protein domain is involved in cell trafficking by aiding import and export of substances in and out of the cell.
Exomer is a heterotetrameric protein complex similar to COPI and other adaptins. It was first described in the yeast Saccharomyces cerevisiae. Exomer is a cargo adaptor important in transporting molecules from the Golgi apparatus toward the cell membrane. The vesicles it is found on are different from COPI vesicles in that they do not appear to have a "coat" or "scaffold" around them.
Halperin-Birk syndrome (HLBKS) is a rare autosomal recessive neurodevelopmental disorder caused by a null mutation in the SEC31A gene. Signs and symptoms include intrauterine growth retardation, marked developmental delay, spastic quadriplegia with profound contractures, dysmorphism, and optic nerve atrophy with no eye fixation. Brain MRI demonstrated microcephaly and agenesis of the corpus callosum.














