Structure
Under normal cellular conditions, cytoplasmic GAPDH exists primarily as a tetramer. This form is composed of four identical 37-kDa subunits containing a single catalytic thiol group each and critical to the enzyme's catalytic function. [6] [7] Nuclear GAPDH has increased isoelectric point (pI) of pH 8.3–8.7. [7] Of note, the cysteine residue C152 in the enzyme's active site is required for the induction of apoptosis by oxidative stress. [7] Notably, post-translational modifications of cytoplasmic GAPDH contribute to its functions outside of glycolysis. [6]
GAPDH is encoded by a single gene that produces a single mRNA transcript with 8 splice variants, though an isoform does exist as a separate gene that is expressed only in spermatozoa. [7]
Reaction
Compound C00118 at KEGG Pathway Database.Enzyme 1.2.1.12 at KEGG Pathway Database.Reaction R01063 at KEGG Pathway Database.Compound C00236 at KEGG Pathway Database.
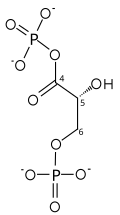
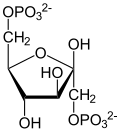
Two-step conversion of G3P
The first reaction is the oxidation of glyceraldehyde 3-phosphate (G3P) at the position-1 (in the diagram it is shown as the 4th carbon from glycolysis), in which an aldehyde is converted into a carboxylic acid (ΔG°'=-50 kJ/mol (−12kcal/mol)) and NAD+ is simultaneously reduced endergonically to NADH.
The energy released by this highly exergonic oxidation reaction drives the endergonic second reaction (ΔG°'=+50 kJ/mol (+12kcal/mol)), in which a molecule of inorganic phosphate is transferred to the GAP intermediate to form a product with high phosphoryl-transfer potential: 1,3-bisphosphoglycerate (1,3-BPG).
This is an example of phosphorylation coupled to oxidation, and the overall reaction is somewhat endergonic (ΔG°'=+6.3 kJ/mol (+1.5)). Energy coupling here is made possible by GAPDH.
Mechanism
GAPDH uses covalent catalysis and general base catalysis to decrease the very large activation energy of the second step (phosphorylation) of this reaction.
1: Oxidation
First, a cysteine residue in the active site of GAPDH attacks the carbonyl group of G3P, creating a hemithioacetal intermediate (covalent catalysis).
The hemithioacetal is deprotonated by a histidine residue in the enzyme's active site (general base catalysis). Deprotonation encourages the reformation of the carbonyl group in the subsequent thioester intermediate and ejection of a hydride ion.
Next, an adjacent, tightly bound molecule of NAD+ accepts the hydride ion, forming NADH while the hemithioacetal is oxidized to a thioester.
This thioester species is much higher in energy (less stable) than the carboxylic acid species that would result if G3P were oxidized in the absence of GAPDH (the carboxylic acid species is so low in energy that the energy barrier for the second step of the reaction (phosphorylation) would be too high, and the reaction, therefore, too slow and unfavorable for a living organism).
2: Phosphorylation
NADH leaves the active site and is replaced by another molecule of NAD+, the positive charge of which stabilizes the negatively charged carbonyl oxygen in the transition state of the next and ultimate step. Finally, a molecule of inorganic phosphate attacks the thioester and forms a tetrahedral intermediate, which then collapses to release 1,3-bisphosphoglycerate, and the thiol group of the enzyme's cysteine residue.
Function
As its name indicates, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) catalyses the conversion of glyceraldehyde 3-phosphate to D-glycerate 1,3-bisphosphate. This is the 6th step in the glycolytic breakdown of glucose, an important pathway of energy and carbon molecule supply which takes place in the cytosol of eukaryotic cells. The conversion occurs in two coupled steps. The first is favourable and allows the second unfavourable step to occur.
Transcription and apoptosis
GAPDH can itself activate transcription. The OCA-S transcriptional coactivator complex contains GAPDH and lactate dehydrogenase, two proteins previously only thought to be involved in metabolism. GAPDH moves between the cytosol and the nucleus and may thus link the metabolic state to gene transcription. [18]
In 2005, Hara et al. showed that GAPDH initiates apoptosis. This is not a third function, but can be seen as an activity mediated by GAPDH binding to DNA like in transcription activation, discussed above. The study demonstrated that GAPDH is S-nitrosylated by NO in response to cell stress, which causes it to bind to the protein SIAH1, a ubiquitin ligase. The complex moves into the nucleus where Siah1 targets nuclear proteins for degradation, thus initiating controlled cell shutdown. [19] In subsequent study the group demonstrated that deprenyl, which has been used clinically to treat Parkinson's disease, strongly reduces the apoptotic action of GAPDH by preventing its S-nitrosylation and might thus be used as a drug. [20]
GAPDH acts as a reversible metabolic switch under oxidative stress. [21] When cells are exposed to oxidants, they need excessive amounts of the antioxidant cofactor NADPH. In the cytosol, NADPH is reduced from NADP+ by several enzymes, three of them catalyze the first steps of the pentose phosphate pathway. Oxidant-treatments cause an inactivation of GAPDH. This inactivation re-routes temporally the metabolic flux from glycolysis to the pentose phosphate pathway, allowing the cell to generate more NADPH. [22] Under stress conditions, NADPH is needed by some antioxidant-systems including glutaredoxin and thioredoxin as well as being essential for the recycling of gluthathione.
Additional functions
GAPDH, like many other enzymes, has multiple functions. In addition to catalysing the 6th step of glycolysis, recent evidence implicates GAPDH in other cellular processes. GAPDH has been described to exhibit higher order multifunctionality in the context of maintaining cellular iron homeostasis, [24] specifically as a chaperone protein for labile heme within cells. [25] This came as a surprise to researchers but it makes evolutionary sense to re-use and adapt existing proteins instead of evolving a novel protein from scratch.
Clinical significance
Cancer
GAPDH is overexpressed in multiple human cancers, such as cutaneous melanoma, and its expression is positively correlated with tumor progression. [32] [33] Its glycolytic and antiapoptotic functions contribute to proliferation and protection of tumor cells, promoting tumorigenesis. Notably, GAPDH protects against telomere shortening induced by chemotherapeutic drugs that stimulate the sphingolipid ceramide. Meanwhile, conditions like oxidative stress impair GAPDH function, leading to cellular aging and death. [7] Moreover, depletion of GAPDH has managed to induce senescence in tumor cells, thus presenting a novel therapeutic strategy for controlling tumor growth. [34]
Neurodegeneration
GAPDH has been implicated in several neurodegenerative diseases and disorders, largely through interactions with other proteins specific to that disease or disorder. These interactions may affect not only energy metabolism but also other GAPDH functions. [6] For example, GAPDH interactions with beta-amyloid precursor protein (betaAPP) could interfere with its function regarding the cytoskeleton or membrane transport, while interactions with huntingtin could interfere with its function regarding apoptosis, nuclear tRNA transport, DNA replication, and DNA repair. In addition, nuclear translocation of GAPDH has been reported in Parkinson's disease (PD), and several anti-apoptotic PD drugs, such as rasagiline, function by preventing the nuclear translocation of GAPDH. It is proposed that hypometabolism may be one contributor to PD, but the exact mechanisms underlying GAPDH involvement in neurodegenerative disease remains to be clarified. [35] The SNP rs3741916 in the 5' UTR of the GAPDH gene may be associated with late onset Alzheimer's disease. [36]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.