
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is the metabolite that occurs as an intermediate in several central pathways of all organisms. With the chemical formula H(O)CCH(OH)CH2OPO32-, this anion is a monophosphate ester of glyceraldehyde.
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis. It is the phosphate ester of dihydroxyacetone.
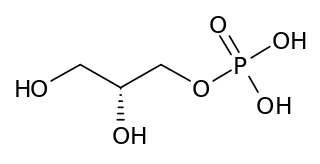
sn-Glycerol 3-phosphate is a phosphoric ester of glycerol, which is a component of glycerophospholipids. Equally appropriate names in biochemical context include glycerol-3-phosphate, 3-O-phosphonoglycerol, 3-phosphoglycerol; and Gro3P. From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid. It should not be confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate.
In enzymology, a [3-methyl-2-oxobutanoate dehydrogenase (acetyl-transferring)] is an enzyme that catalyzes the chemical reaction
In enzymology, a [acetyl-CoA carboxylase] kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an acylglycerol kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an alkylglycerol kinase is an enzyme that catalyzes the chemical reaction

In enzymology, a guanylate kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a [isocitrate dehydrogenase (NADP+)] kinase (EC 2.7.11.5) is an enzyme that catalyzes the chemical reaction:
Aminoglycoside-3'-phosphotransferase, also known as aminoglycoside kinase, is an enzyme that primarily catalyzes the addition of phosphate from ATP to the 3'-hydroxyl group of a 4,6-disubstituted aminoglycoside, such as kanamycin. However, APH(3') has also been found to phosphorylate at the 5'-hydroxyl group in 4,5-disubstituted aminoglycosides, which lack a 3'-hydroxyl group, and to diphosphorylate hydroxyl groups in aminoglycosides that have both 3'- and 5'-hydroxyl groups. Primarily positively charged at biological conditions, aminoglycosides bind to the negatively charged backbone of nucleic acids to disrupt protein synthesis, effectively inhibiting bacterial cell growth. APH(3') mediated phosphorylation of aminoglycosides effectively disrupts their mechanism of action, introducing a phosphate group that reduces their binding affinity due to steric hindrances and unfavorable electrostatic interactions. APH(3') is primarily found in certain species of gram-positive bacteria.
In enzymology, a low-density-lipoprotein receptor kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine pros-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine tele-kinase is an enzyme that catalyzes the chemical reaction

Shikimate kinase is an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate. This reaction is the fifth step of the shikimate pathway, which is used by plants and bacteria to synthesize the common precursor of aromatic amino acids and secondary metabolites. The systematic name of this enzyme class is ATP:shikimate 3-phosphotransferase. Other names in common use include shikimate kinase (phosphorylating), and shikimate kinase II.
In enzymology, a thiamine-diphosphate kinase is an enzyme involved in thiamine metabolism. It catalyzes the chemical reaction
In enzymology, a [tyrosine 3-monooxygenase] kinase is an enzyme that catalyzes the chemical reaction
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Unlike glucose, which is directly metabolized widely in the body, fructose is almost entirely metabolized in the liver in humans, where it is directed toward replenishment of liver glycogen and triglyceride synthesis. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.
Glycerol kinase deficiency (GKD) is an X-linked recessive enzyme defect that is heterozygous in nature. Three clinically distinct forms of this deficiency have been proposed, namely infantile, juvenile, and adult. National Institutes of Health and its Office of Rare Diseases Research branch classifies GKD as a rare disease, known to affect fewer than 200,000 individuals in the United States. The responsible gene lies in a region containing genes in which deletions can cause Duchenne muscular dystrophy and adrenal hypoplasia congenita. Combinations of these three genetic defects including GKD are addressed medically as Complex GKD.





