| Phosphofructokinase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | Ppfruckinase | ||||||||||
| Pfam | PF00365 | ||||||||||
| InterPro | IPR000023 | ||||||||||
| PROSITE | PDOC00336 | ||||||||||
| |||||||||||
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
| Phosphofructokinase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | Ppfruckinase | ||||||||||
| Pfam | PF00365 | ||||||||||
| InterPro | IPR000023 | ||||||||||
| PROSITE | PDOC00336 | ||||||||||
| |||||||||||
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. [1] Phosphofructokinase catalyses the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate, a key regulatory step in the glycolytic pathway. [2] [3] It is allosterically inhibited by ATP and allosterically activated by AMP, thus indicating the cell's energetic needs when it undergoes the glycolytic pathway. [4] PFK exists as a homotetramer in bacteria and mammals (where each monomer possesses 2 similar domains) and as an octomer in yeast (where there are 4 alpha- (PFK1) and 4 beta-chains (PFK2), the latter, like the mammalian monomers, possessing 2 similar domains [3] ). This protein may use the morpheein model of allosteric regulation. [5]
PFK is about 300 amino acids in length, and structural studies of the bacterial enzyme have shown it comprises two similar (alpha/beta) lobes: one involved in ATP binding and the other housing both the substrate-binding site and the allosteric site (a regulatory binding site distinct from the active site, but that affects enzyme activity). The identical tetramer subunits adopt 2 different conformations: in a 'closed' state, the bound magnesium ion bridges the phosphoryl groups of the enzyme products (ADP and fructose-1,6-bisphosphate); and in an 'open' state, the magnesium ion binds only the ADP, [6] as the 2 products are now further apart. These conformations are thought to be successive stages of a reaction pathway that requires subunit closure to bring the 2 molecules sufficiently close to react. [6]
The reverse reaction is catalyzed by the enzyme Fructose-1,6-bisphosphatase.[ citation needed ]
PFK belongs to the phosphofructokinase B (PfkB) family of sugar kinases. [7] Other members of this family (also known as the Ribokinase family) include ribokinase (RK), adenosine kinase (AK), inosine kinase, and 1-phosphofructokinase. [7] [8] [9] The members of the PfkB/RK family are identified by the presence of three conserved sequence motifs. [7] [8] [10] The structures of several PfK family of proteins have been determined from a number of organisms and the enzymatic activity of this family of protein shows a dependence on the presence of pentavalent ions. [11] [7] [10] PFK is found in isoform versions in skeletal muscle (PFKM), in the liver (PFKL), and from platelets (PFKP), allowing for tissue-specific expression and function. It is still speculated that the isoforms may play a role in specific glycolytic rates in the tissue-specific environments they are in. It has been found in humans that some human tumor cell lines had increased glycolytic productivity and correlated with the increased amount of PFKL. [12] [13]
Deficiency in PFK leads to glycogenosis type VII (Tarui's disease), an autosomal recessive disorder characterised by severe nausea, vomiting, muscle cramps and myoglobinuria in response to bursts of intense or vigorous exercise. [3] Sufferers are usually able to lead a reasonably ordinary life by learning to adjust activity levels. [3]
There are two different phosphofructokinase enzymes in humans:
| Type | Synonyms | EC number | Substrate | Product | Paralog genes |
|---|---|---|---|---|---|
| Phosphofructokinase 1 | 6-phosphofructokinase phosphohexokinase | EC 2.7.1.11 |  Fructose 6-phosphate |  Fructose-1,6-bisphosphate | PFKL, PFKM, PFKP |
| Phosphofructokinase 2 | 6-phosphofructo-2-kinase | EC 2.7.1.105 | 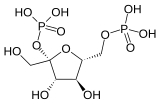 Fructose-2,6-bisphosphate | PFKFB1, PFKFB2, PFKFB3, PFKFB4 |

Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

Glycolysis is the metabolic pathway that converts glucose into pyruvate. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

Diphosphate—fructose-6-phosphate 1-phosphotransferase also known as PFP is an enzyme of carbohydrate metabolism in plants and some bacteria. The enzyme catalyses the reversible interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate using inorganic pyrophosphate as the phosphoryl donor:

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

Phosphofructokinase deficiency is a rare muscular metabolic disorder, with an autosomal recessive inheritance pattern.
Adenylate kinase is a phosphotransferase enzyme that catalyzes the interconversion of the various adenosine phosphates. By constantly monitoring phosphate nucleotide levels inside the cell, ADK plays an important role in cellular energy homeostasis.

Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose and mannose metabolism. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases, however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.

UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,

Fructose 2,6-bisphosphate, abbreviated Fru-2,6-P2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-P2 itself is synthesized and broken down by the bifunctional enzyme phosphofructokinase 2/fructose-2,6-bisphosphatase (PFK-2/FBPase-2).
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

In enzymology, 1-phosphofructokinase is an enzyme that catalyzes the chemical reaction

Adenosine kinase is an enzyme that catalyzes the transfer of gamma-phosphate from Adenosine triphosphate (ATP) to adenosine (Ado) leading to formation of Adenosine monophosphate (AMP). In addition to its well-studied role in controlling the cellular concentration of Ado, AdK also plays an important role in the maintenance of methylation reactions. All S-adenosylmethionine-dependent transmethylation reactions in cells lead to production of S-adenosylhomocysteine (SAH), which is cleaved by SAH hydrolase into Ado and homocysteine. The failure to efficiently remove these end products can result in buildup of SAH, which is a potent inhibitor of all transmethylation reactions. The disruption of AdK gene (-/-) in mice causes neonatal hepatic steatosis, a fatal condition characterized by rapid microvesicular fat infiltration, leading to early postnatal death. The liver was the main organ affected in these animals and in it the levels of adenine nucleotides were decreased, while those of SAH were elevated. Recently, missense mutations in the AdK gene in humans which result in AdK deficiency have also been shown to cause hypermethioninemia, encephalopathy and abnormal liver function.

In enzymology, an inosine kinase is an enzyme that catalyzes the chemical reaction

In enzymology, a ribokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a tagatose-6-phosphate kinase is an enzyme that catalyzes the chemical reaction

6-phosphofructokinase, muscle type is an enzyme that in humans is encoded by the PFKM gene on chromosome 12. Three phosphofructokinase isozymes exist in humans: muscle, liver and platelet. These isozymes function as subunits of the mammalian tetramer phosphofructokinase, which catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. Tetramer composition varies depending on tissue type. This gene encodes the muscle-type isozyme. Mutations in this gene have been associated with glycogen storage disease type VII, also known as Tarui disease. Alternatively spliced transcript variants have been described.[provided by RefSeq, Nov 2009]

PFKFB3 is a gene that encodes the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 enzyme in humans. It is one of 4 tissue-specific PFKFB isoenzymes identified currently (PFKFB1-4).

6-phosphofructokinase, liver type (PFKL) is an enzyme that in humans is encoded by the PFKL gene on chromosome 21. This gene encodes the liver (L) subunit of an enzyme that catalyzes the conversion of D-fructose 6-phosphate to D-fructose 1,6-bisphosphate, which is a key step in glucose metabolism (glycolysis). This enzyme is a tetramer that may be composed of different subunits encoded by distinct genes in different tissues. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Mar 2014]

Phosphofructokinase, platelet, also known as PFKP is an enzyme which in humans is encoded by the PFKP gene.