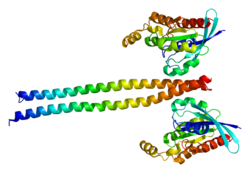Angiomotin (AMOT) is a protein that in humans is encoded by the AMOT gene. It belongs to the motin family of angiostatin binding proteins, which includes angiomotin, angiomotin-like 1 (AMOTL1) and angiomotin-like 2 (AMOTL2) characterized by coiled-coil domains at N-terminus and consensus PDZ-binding domain at the C-terminus. Angiomotin is expressed predominantly in endothelial cells of capillaries as well as angiogenic tissues such as placenta and solid tumor.
The Rho family of GTPases is a family of small signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions.

Cell division control protein 42 homolog is a protein that in humans is encoded by the CDC42 gene. Cdc42 is involved in regulation of the cell cycle. It was originally identified in S. cerevisiae (yeast) as a mediator of cell division, and is now known to influence a variety of signaling events and cellular processes in a variety of organisms from yeast to mammals.

Ras-related C3 botulinum toxin substrate 1, is a protein that in humans is encoded by the RAC1 gene. This gene can produce a variety of alternatively spliced versions of the Rac1 protein, which appear to carry out different functions.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility.

Serine/threonine-protein kinase PAK 1 is an enzyme that in humans is encoded by the PAK1 gene.

LIM domain kinase 1 is an enzyme that in humans is encoded by the LIMK1 gene.

Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.

RhoC is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RHOC.

Serine/threonine-protein kinase PAK 4 is an enzyme that in humans is encoded by the PAK4 gene.

Deleted in Liver Cancer 1 also known as DLC1 and StAR-related lipid transfer protein 12 (STARD12) is a protein which in humans is encoded by the DLC1 gene.

Rnd3 is a small signaling G protein, and is a member of the Rnd subgroup of the Rho family of GTPases. It is encoded by the gene RND3.

Cyclin-dependent kinase 5 is a protein, and more specifically an enzyme, that is encoded by the Cdk5 gene. It was discovered 15 years ago, and it is saliently expressed in post-mitotic central nervous system neurons (CNS).
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

Dishevelled (Dsh) is a family of proteins involved in canonical and non-canonical Wnt signalling pathways. Dsh is a cytoplasmic phosphoprotein that acts directly downstream of frizzled receptors. It takes its name from its initial discovery in flies, where a mutation in the dishevelled gene was observed to cause improper orientation of body and wing hairs. There are vertebrate homologs in zebrafish, Xenopus (Xdsh), mice and humans. Dsh relays complex Wnt signals in tissues and cells, in normal and abnormal contexts. It is thought to interact with the SPATS1 protein when regulating the Wnt Signalling pathway.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.

In molecular biology, the IMD domain is a BAR-like domain of approximately 250 amino acids found at the N-terminus in the insulin receptor tyrosine kinase substrate p53 (IRSp53/BAIAP2) and in the evolutionarily related IRSp53/MIM (MTSS1) family. In IRSp53, a ubiquitous regulator of the actin cytoskeleton, the IMD domain acts as conserved F-actin bundling domain involved in filopodium formation. Filopodium-inducing IMD activity is regulated by Cdc42 and Rac1 and is SH3-independent. The IRSp53/MIM family is a novel F-actin bundling protein family that includes invertebrate relatives:

XB130 is a cytosolic adaptor protein and signal transduction mediator. XB130 regulates cell proliferation, cell survival, cell motility and gene expression. XB130 is highly similar to AFAP and is thus known as actin filament associated protein 1-like 2 (AFAP1L2). XB130 is a substrate and regulator of multiple tyrosine kinase-mediated signaling. XB130 is highly expressed in the thyroid and spleen.

Rho-kinase inhibitors are a series of compounds that target rho kinase (ROCK) and inhibit the ROCK pathway. Clinical trials have found that inhibition of the ROCK pathway contributes to the cardiovascular benefits of statin therapy. Furthermore, ROCK inhibitors may have clinical applications for anti-erectile dysfunction, antihypertension, and tumor metastasis inhibition. More recently they have been studied for the treatment of glaucoma and as a therapeutic target for the treatment of cardiovascular diseases, including ischemic stroke. While statin therapy has been demonstrated to reduce the risk of major cardiovascular events, including ischemic stroke, the interplay between the ROCK pathway and statin therapy to treat and prevent strokes in older adults has not yet been proven.