Related Research Articles

An axon or nerve fiber is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.
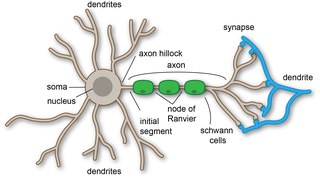
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

A neuron, neurone, or nerve cell is an excitable cell that fires electric signals called action potentials across a neural network in the nervous system. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

In cellular neuroscience, the soma, perikaryon, neurocyton, or cell body is the bulbous, non-process portion of a neuron or other brain cell type, containing the cell nucleus. Although it is often used to refer to neurons, it can also refer to other cell types as well, including astrocytes, oligodendrocytes, and microglia. There are many different specialized types of neurons, and their sizes vary from as small as about 5 micrometres to over 10 millimetres for some of the smallest and largest neurons of invertebrates, respectively.
Axon guidance is a subfield of neural development concerning the process by which neurons send out axons to reach their correct targets. Axons often follow very precise paths in the nervous system, and how they manage to find their way so accurately is an area of ongoing research.
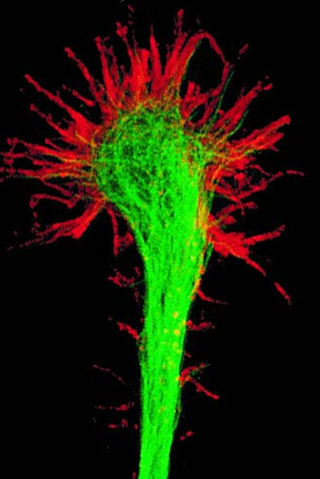
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements". Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure.
Neuroregeneration involves the regrowth or repair of nervous tissues, cells or cell products. Neuroregenerative mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.

The subcommissural organ (SCO) is one of the circumventricular organs of the brain. It is a small glandular structure that is located in the posterior region of the third ventricle, near the entrance of the cerebral aqueduct.

A glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.

Contactin 1, also known as CNTN1, is a protein which in humans is encoded by the CNTN1 gene.

Receptor-type tyrosine-protein phosphatase mu is an enzyme that in humans is encoded by the PTPRM gene.
Collapsin response mediator protein family or CRMP family consists of five intracellular phosphoproteins of similar molecular size and high (50–70%) amino acid sequence identity. CRMPs are predominantly expressed in the nervous system during development and play important roles in axon formation from neurites and in growth cone guidance and collapse through their interactions with microtubules. Cleaved forms of CRMPs have also been linked to neuron degeneration after trauma induced injury.

Cellular adhesions can be defined as proteins or protein aggregates that form mechanical and chemical linkages between the intracellular and extracellular space. Adhesions serve several critical processes including cell migration, signal transduction, tissue development and repair. Due to this functionality, adhesions and adhesion molecules have been a topic of study within the scientific community. Specifically, it has been found that adhesions are involved in tissue development, plasticity, and memory formation within the central nervous system (CNS), and may prove vital in the generation of CNS-specific therapeutics.
Fasciclin 2 is a 95 kilodalton cell membrane glycoprotein in the immunoglobulin (Ig) – related superfamily of cell adhesion molecules (CAMs). It was first identified in the developing grasshopper embryo, seen dynamically expressed on a subset of fasciculating axons in the central nervous system (CNS), functioning as a neuronal recognition molecule in the regulation of selective axon fasciculation. Subsequently, fasII was cloned and has mainly been studied in the fruit fly. Its extracellular structure consists of two Fibronectin type III domains and five Ig-like C2 domains, having structural homology to the neural cell adhesion molecule (NCAM) found in vertebrates. Alternative splicing of fasII gives rise to its expression in three major isoforms, including a membrane-associated form that is attached to the outer leaflet of the plasma membrane via a glycophosphatidylinositol linkage and two integral transmembrane forms. The larger transmembrane form has an amino acid motif contained in its cytoplasmic domain that is rich in proline, glutamic acid, serine and threonine residues. The fasciclin 1 (Fas1) and fasciclin 3 (Fas3) genes in Drosophila also code for cell adhesion proteins in the nervous system but do not show any structural or functional similarities with NCAM.

Neuronal self-avoidance, or isoneural avoidance, is an important property of neurons which consists in the tendency of branches arising from a single soma to turn away from one another. The arrangements of branches within neuronal arbors are established during development and result in minimal crossing or overlap as they spread over a territory, resulting in the typical fasciculated morphology of neurons.
N2a cells are a fast-growing mouse neuroblastoma cell line.

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.
References
- ↑ Flynn, Kevin C (2013-01-01). "The cytoskeleton and neurite initiation". Bioarchitecture. 3 (4): 86–109. doi:10.4161/bioa.26259. ISSN 1949-0992. PMC 4201609 . PMID 24002528.
- 1 2 3 Valtorta, F.; Leoni, C. (1999-02-28). "Molecular mechanisms of neurite extension". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 354 (1381): 387–394. doi:10.1098/rstb.1999.0391. ISSN 0962-8436. PMC 1692490 . PMID 10212488.
- ↑ Niclou, Simone P.; Franssen, Elske H. P.; Ehlert, Erich M. E.; Taniguchi, Masahiko; Verhaagen, Joost (2003-12-01). "Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth" (PDF). Molecular and Cellular Neurosciences. 24 (4): 902–912. doi:10.1016/s1044-7431(03)00243-4. ISSN 1044-7431. PMID 14697657. S2CID 12637023.
- ↑ Luo, Y.; Raible, D.; Raper, J. A. (1993-10-22). "Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones". Cell. 75 (2): 217–227. doi: 10.1016/0092-8674(93)80064-l . ISSN 0092-8674. PMID 8402908. S2CID 46120825.
- ↑ Bear, Mark F; Connors, Barry W.; Paradiso, Michael A., Neuroscience, Exploring the Brain, Philadelphia : Lippincott Williams & Wilkins; Third Edition (February 1, 2006). ISBN 0-7817-6003-8
- ↑ Qiang, Liang; Yu, Wenqian; Andreadis, Athena; Luo, Minhua; Baas, Peter W. (22 March 2006). "Tau Protects Microtubules in the Axon from Severing by Katanin". The Journal of Neuroscience. 26 (12): 3120–3129. doi:10.1523/JNEUROSCI.5392-05.2006. ISSN 0270-6474. PMC 6674103 . PMID 16554463.
- ↑ Xiao, Yangui; Peng, Yinghui; Wan, Jun; Tang, Genyun; Chen, Yuewen; Tang, Jing; Ye, Wen-Cai; Ip, Nancy Y.; Shi, Lei (2013-07-05). "The Atypical Guanine Nucleotide Exchange Factor Dock4 Regulates Neurite Differentiation through Modulation of Rac1 GTPase and Actin Dynamics". Journal of Biological Chemistry. 288 (27): 20034–20045. doi: 10.1074/jbc.M113.458612 . ISSN 0021-9258. PMC 3707701 . PMID 23720743.
- ↑ Toriyama, Michinori; Kozawa, Satoshi; Sakumura, Yuichi; Inagaki, Naoyuki (2013-03-18). "Conversion of a signal into forces for axon outgrowth through Pak1-mediated shootin1 phosphorylation". Current Biology. 23 (6): 529–534. Bibcode:2013CBio...23..529T. doi: 10.1016/j.cub.2013.02.017 . hdl: 10061/8621 . ISSN 1879-0445. PMID 23453953.
- ↑ Berezin, Vladimir (2009-12-17). Structure and Function of the Neural Cell Adhesion Molecule NCAM. Springer Science & Business Media. ISBN 978-1-4419-1170-4.
- ↑ "NeuronJ". imagescience.org. Retrieved 2024-06-10.
- ↑ Myatt, Darren R.; Hadlington, Tye; Ascoli, Giorgio A.; Nasuto, Slawomir J. (2012-03-16). "Neuromantic – from Semi-Manual to Semi-Automatic Reconstruction of Neuron Morphology". Frontiers in Neuroinformatics. 6: 4. doi: 10.3389/fninf.2012.00004 . ISSN 1662-5196. PMC 3305991 . PMID 22438842.
- ↑ "Neurolucida®". MBF Bioscience. Retrieved 2024-06-10.
- ↑ Jones, Peter D.; Molina-Martínez, Beatriz; Niedworok, Anita; Cesare, Paolo (2024). "A microphysiological system for parallelized morphological and electrophysiological read-out of 3D neuronal cell culture". Lab on a Chip. 24 (6): 1750–1761. doi:10.1039/D3LC00963G. ISSN 1473-0197. PMID 38348692.
- 1 2 3 4 5 6 7 8 9 10 11 Takano, Tetsuya; Xu, Chundi; Funahashi, Yasuhiro; Namba, Takashi; Kaibuchi, Kozo (2015-06-15). "Neuronal polarization". Development. 142 (12): 2088–2093. doi: 10.1242/dev.114454 . ISSN 0950-1991. PMID 26081570.
- ↑ Arimura, Nariko; Kaibuchi, Kozo (2007-03-01). "Neuronal polarity: from extracellular signals to intracellular mechanisms". Nature Reviews Neuroscience. 8 (3): 194–205. doi:10.1038/nrn2056. ISSN 1471-003X. PMID 17311006. S2CID 15556921.
- 1 2 Inagaki, Naoyuki; Toriyama, Michinori; Sakumura, Yuichi (2011-06-01). "Systems biology of symmetry breaking during neuronal polarity formation". Developmental Neurobiology. 71 (6): 584–593. doi:10.1002/dneu.20837. hdl: 10061/10669 . ISSN 1932-846X. PMID 21557507. S2CID 14746741.