Protein kinase C delta type (or PKC-δ) is an enzyme that in humans is encoded by the PRKCD gene. [5]
Protein kinase C delta type (or PKC-δ) is an enzyme that in humans is encoded by the PRKCD gene. [5]
Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by the second messenger diacylglycerol. [6] PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play distinct roles in cells. The protein encoded by this gene is one of the PKC family members. Studies both in human and mice demonstrate that this kinase is involved in B cell signaling and in the regulation of growth, apoptosis, and differentiation of a variety of cell types. [7] Protein kinase C delta is also regulated by phosphorylation on various serine/threonine (e.g. T50, T141, S304, T451, T505, S506, T507, S643, S664) and tyrosine residues including Y311 (by SRC). [8] [9]
PRKCD has been shown to interact with:

Tyrosine-protein kinase ABL1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene, which was initially isolated from the Abelson murine leukemia virus.

Dual specificity mitogen-activated protein kinase kinase 1 is an enzyme that in humans is encoded by the MAP2K1 gene.
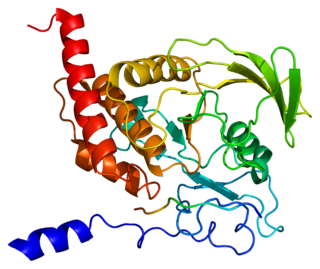
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the PTPN6 gene.

AKT2, also known as RAC-beta serine/threonine-protein kinase, is an enzyme that in humans is encoded by the AKT2 gene. It influences metabolite storage as part of the insulin signal transduction pathway.

Mitogen-activated protein kinase 7 also known as MAP kinase 7 is an enzyme that in humans is encoded by the MAPK7 gene.

Protein tyrosine kinase 2 beta is an enzyme that in humans is encoded by the PTK2B gene.

Mitogen-activated protein kinase kinase kinase 11 is an enzyme that in humans is encoded by the MAP3K11 gene.
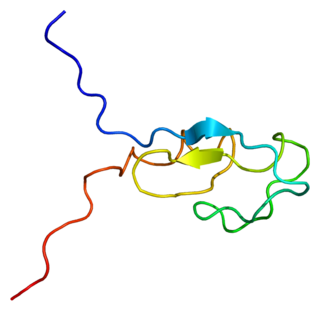
Protein kinase C gamma type is an enzyme that in humans is encoded by the PRKCG gene.
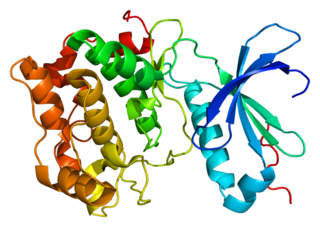
Protein kinase C theta (PKC-θ) is an enzyme that in humans is encoded by the PRKCQ gene. PKC-θ, a member of serine/threonine kinases, is mainly expressed in hematopoietic cells with high levels in platelets and T lymphocytes, where plays a role in signal transduction. Different subpopulations of T cells vary in their requirements of PKC-θ, therefore PKC-θ is considered as a potential target for inhibitors in the context of immunotherapy.

Serine/threonine-protein kinase D1 is an enzyme that in humans is encoded by the PRKD1 gene.

Protein kinase C eta type is an enzyme that in humans is encoded by the PRKCH gene.

Tyrosine-protein kinase ABL2 also known as Abelson-related gene (Arg) is an enzyme that in humans is encoded by the ABL2 gene.

Serine/threonine-protein kinase D3 (PKD3) or PKC-nu is an enzyme that in humans is encoded by the PRKD3 gene.

Polo-like kinase 3 (Drosophila), also known as PLK3, is an enzyme which in humans is encoded by the PLK3 gene.

Serine/threonine-protein kinase N2 is an enzyme that in humans and Strongylocentrotus purpuratus is encoded by the PKN2 gene.

MAP kinase-interacting serine/threonine-protein kinase 1 is an enzyme that in humans is encoded by the MKNK1 gene.

Ribosomal protein S6 kinase beta-2 is an enzyme that in humans is encoded by the RPS6KB2 gene.

Ribosomal protein S6 kinase alpha-4 is an enzyme that in humans is encoded by the RPS6KA4 gene.

Diacylglycerol kinase delta is an enzyme that in humans is encoded by the DGKD gene.

BIM-1 and the related compounds BIM-2, BIM-3, and BIM-8 are bisindolylmaleimide-based protein kinase C (PKC) inhibitors. These inhibitors also inhibit PDK1 explaining the higher inhibitory potential of LY33331 compared to the other BIM compounds a bisindolylmaleimide inhibitor toward PDK1.