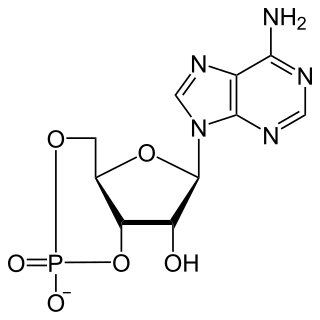
Cyclic adenosine monophosphate is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

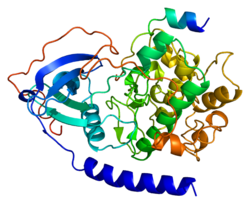
In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.

Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
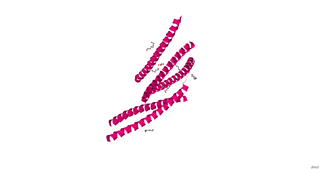
cGMP-dependent protein kinase or protein kinase G (PKG) is a serine/threonine-specific protein kinase that is activated by cGMP. It phosphorylates a number of biologically important targets and is implicated in the regulation of smooth muscle relaxation, platelet function, sperm metabolism, cell division, and nucleic acid synthesis.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.

Phosphorylase kinase (PhK) is a serine/threonine-specific protein kinase which activates glycogen phosphorylase to release glucose-1-phosphate from glycogen. PhK phosphorylates glycogen phosphorylase at two serine residues, triggering a conformational shift which favors the more active glycogen phosphorylase “a” form over the less active glycogen phosphorylase b.

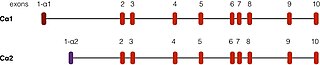
cAMP-dependent protein kinase type I-alpha regulatory subunit is an enzyme that in humans is encoded by the PRKAR1A gene.

cAMP-dependent protein kinase type II-alpha regulatory subunit is an enzyme that in humans is encoded by the PRKAR2A gene.

cAMP-dependent protein kinase type II-beta regulatory subunit is an enzyme that in humans is encoded by the PRKAR2B gene.

A-kinase anchor protein 5 is a protein that in humans is encoded by the AKAP5 gene.

cAMP-dependent protein kinase catalytic subunit beta is an enzyme that in humans is encoded by the PRKACB gene.

A-kinase anchor protein 12, aka AKAP250, is an enzyme that in humans is encoded by the AKAP12 gene.

cAMP-dependent protein kinase type I-beta regulatory subunit is an enzyme that in humans is encoded by the PRKAR1B gene.

A kinase anchor protein 1, mitochondrial is an enzyme that in humans is encoded by the AKAP1 gene.

A-kinase anchor protein 8 is an enzyme that, in humans, is encoded by the AKAP8 gene.

cAMP-dependent protein kinase catalytic subunit gamma is an enzyme that in humans is encoded by the PRKACG gene.

A-kinase anchor protein 7 isoform gamma is an enzyme that in humans is encoded by the AKAP7 gene.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.

Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis, cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.