
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

The citric acid cycle —also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.
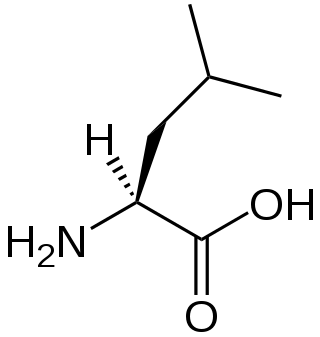
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.
The branched-chain α-ketoacid dehydrogenase complex is a multi-subunit complex of enzymes that is found on the mitochondrial inner membrane. This enzyme complex catalyzes the oxidative decarboxylation of branched, short-chain alpha-ketoacids. BCKDC is a member of the mitochondrial α-ketoacid dehydrogenase complex family comprising pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, key enzymes that function in the Krebs cycle.

A serine/threonine protein kinase is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK).

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
In enzymology, a 2-oxoisovalerate dehydrogenase (acylating) (EC 1.2.1.25) is an enzyme that catalyzes the chemical reaction

In enzymology, a 3-methyl-2-oxobutanoate dehydrogenase (EC 1.2.4.4) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-methyl-2-oxobutanoate dehydrogenase (ferredoxin) (EC 1.2.7.7) is an enzyme that catalyzes the chemical reaction

In enzymology, an aspartate-semialdehyde dehydrogenase is an enzyme that is very important in the biosynthesis of amino acids in prokaryotes, fungi, and some higher plants. It forms an early branch point in the metabolic pathway forming lysine, methionine, leucine and isoleucine from aspartate. This pathway also produces diaminopimelate which plays an essential role in bacterial cell wall formation. There is particular interest in ASADH as disabling this enzyme proves fatal to the organism giving rise to the possibility of a new class of antibiotics, fungicides, and herbicides aimed at inhibiting it.
In enzymology, a [acetyl-CoA carboxylase] kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a Goodpasture-antigen-binding protein kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a tropomyosin kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a [tyrosine 3-monooxygenase] kinase is an enzyme that catalyzes the chemical reaction

A 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial is an enzyme that in humans is encoded by the BCKDHA gene.

Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial is an enzyme that in humans is encoded by the DBT gene.

2-Oxoisovalerate dehydrogenase subunit beta, mitochondrial is an enzyme that in humans is encoded by the BCKDHB gene.

Branched chain ketoacid dehydrogenase kinase (BCKDK) is an enzyme encoded by the BCKDK gene on chromosome 16. This enzyme is part of the mitochondrial protein kinases family and it is a regulator of the valine, leucine, and isoleucine catabolic pathways. BCKDK is found in the mitochondrial matrix and the prevalence of it depends on the type of cell. Liver cells tend to have the lowest concentration of BCKDK, whereas skeletal muscle cells have the highest amount. Abnormal activity of this enzyme often leads to diseases such as maple syrup urine disease and cachexia.
(3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring))-phosphatase (EC 3.1.3.52, branched-chain oxo-acid dehydrogenase phosphatase, branched-chain 2-keto acid dehydrogenase phosphatase, branched-chain α-keto acid dehydrogenase phosphatase, BCKDH', [3-methyl-2-oxobutanoate dehydrogenase (lipoamide)]-phosphatase, [3-methyl-2-oxobutanoate dehydrogenase (lipoamide)]-phosphate phosphohydrolase) is an enzyme with systematic name (3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring))-phosphate phosphohydrolase. This enzyme catalyses the following chemical reaction











