Serine/threonine-protein kinase PLK4 also known as polo-like kinase 4 is an enzyme that in humans is encoded by the PLK4 gene. [5] The Drosophila homolog is SAK, the C. elegans homolog is zyg-1, and the Xenopus homolog is Plx4. [6]
Serine/threonine-protein kinase PLK4 also known as polo-like kinase 4 is an enzyme that in humans is encoded by the PLK4 gene. [5] The Drosophila homolog is SAK, the C. elegans homolog is zyg-1, and the Xenopus homolog is Plx4. [6]
PLK4 encodes a member of the polo family of serine/threonine protein kinases. The protein localizes to centrioles—complex microtubule-based structures found in centrosomes—and regulates centriole duplication during the cell cycle. [5] Overexpression of PLK4 results in centrosome amplification, and knockdown of PLK4 results in loss of centrosomes. [7] [8]
PLK4 contains an N-terminal kinase domain (residues 12-284) and a C-terminal localization domain (residues 596-898). [9] Other polo-like kinase members contain 2 C-terminal polo box domains (PBD). PLK4 contains these 2 domains in addition to a third PBD, which facilitates oligomerization, targeting, and promotes trans-autophosphorylation, limiting centriole duplication to once per cell cycle. [9]
Inhibitors of the enzymatic activity PLK4 have potential in the treatment of cancer. [10] [11] The PLK4 inhibitor R1530 down regulates the expression of mitotic checkpoint kinase BubR1 that in turn leads to polyploidy rendering cancer cells unstable and more sensitive to cancer chemotherapy. Furthermore, normal cells are resistant to the polyploidy inducing effects of R1530. [12]
Another PLK4 inhibitor, CFI-400945 has demonstrated efficacy in animal models of breast and ovarian cancer. [13] [14]
Another PLK4 inhibitor, centrinone, has been reported to deplete centrioles in human and other vertebrate cell types, which resulted in a p53-dependent cell cycle arrest in G1. [15] Inhibition of PLK4 using a chemical genetic strategy has validated this p53-dependent cell cycle arrest in G1. [16]
PLK4 was also identified as a potential therapeutic target for malignant rhabdoid tumors, medulloblastomas and possibly, other embryonal tumors of the brain. [17] [18] [19] [20]
Documented PLK4 substrates include STIL, GCP6, [21] Hand1, [22] [23] Ect2, [24] FBXW5, [25] and itself (via autophosphorylation). Autophosphorylation of PLK4 results in ubiquitination and subsequent destruction by the proteasome. [26] [27]

In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome provides structure for the cell. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use other structures to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.
Aurora kinases are serine/threonine kinases that are essential for cell proliferation. They are phosphotransferase enzymes that help the dividing cell dispense its genetic materials to its daughter cells. More specifically, Aurora kinases play a crucial role in cellular division by controlling chromatid segregation. Defects in this segregation can cause genetic instability, a condition which is highly associated with tumorigenesis. The first aurora kinases were identified in Drosophila melanogaster, where mutations led to failure of centrosome separation with the monopolar spindles reminiscent of the North Pole, suggesting the name aurora.
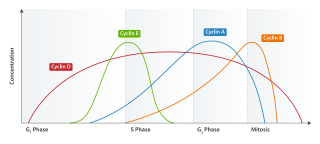
Cyclin E is a member of the cyclin family.

Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.

Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.
David Moore Glover is a British geneticist and Research Professor of Biology and Biological Engineering at the California Institute of Technology. He served as Balfour Professor of Genetics at the University of Cambridge, a Wellcome Trust investigator in the Department of Genetics at the University of Cambridge, and Fellow of Fitzwilliam College, Cambridge. He serves as the first editor-in-chief of the open-access journal Open Biology published by the Royal Society.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Cyclin-dependent kinase 7, or cell division protein kinase 7, is an enzyme that in humans is encoded by the CDK7 gene.

Mitotic checkpoint serine/threonine-protein kinase BUB1 beta is an enzyme that in humans is encoded by the BUB1B gene. Also known as BubR1, this protein is recognized for its mitotic roles in the spindle assembly checkpoint (SAC) and kinetochore-microtubule interactions that facilitate chromosome migration and alignment. BubR1 promotes mitotic fidelity and protects against aneuploidy by ensuring proper chromosome segregation between daughter cells. BubR1 is proposed to prevent tumorigenesis.

Serine/threonine-protein kinase Nek2 is an enzyme that in humans is encoded by the NEK2 gene.

Dual specificity protein kinase TTK also known as Mps1 is an enzyme that in humans is encoded by the TTK gene.

Serine/threonine-protein kinase 38 is an enzyme that in humans is encoded by the STK38 gene.

Centrosomal protein 170kDa, also known as CEP170, is a protein that in humans is encoded by the CEP170 gene.

Centrosomal protein of 192 kDa, also known as Cep192, is a protein that in humans is encoded by the CEP192 gene. It is the homolog of the C. elegans and D. melanogaster gene SPD-2.

Centrosomal protein of 164 kDa, also known as CEP164, is a protein that in humans is encoded by the CEP164 gene. Its function appears two be twofold: CEP164 is required for primary cilium formation. Furthermore, it is an important component in the response to DNA damage by UV light.

Centrosomal protein of 152 kDa, also known as Cep152, is a protein that in humans is encoded by the CEP152 gene. It is the ortholog of the Drosophila melanogaster gene asterless (asl) and both are required for centriole duplication.

Spindle assembly abnormal protein 6 homolog (SAS-6) is a protein that in humans is encoded by the SASS6 gene.

Centrosomes are the major microtubule organizing centers (MTOC) in mammalian cells. Failure of centrosome regulation can cause mistakes in chromosome segregation and is associated with aneuploidy. A centrosome is composed of two orthogonal cylindrical protein assemblies, called centrioles, which are surrounded by a protein dense amorphous cloud of pericentriolar material (PCM). The PCM is essential for nucleation and organization of microtubules. The centrosome cycle is important to ensure that daughter cells receive a centrosome after cell division. As the cell cycle progresses, the centrosome undergoes a series of morphological and functional changes. Initiation of the centrosome cycle occurs early in the cell cycle in order to have two centrosomes by the time mitosis occurs.

Autophosphorylation is a type of post-translational modification of proteins. It is generally defined as the phosphorylation of the kinase by itself. In eukaryotes, this process occurs by the addition of a phosphate group to serine, threonine or tyrosine residues within protein kinases, normally to regulate the catalytic activity. Autophosphorylation may occur when a kinases' own active site catalyzes the phosphorylation reaction, or when another kinase of the same type provides the active site that carries out the chemistry. The latter often occurs when kinase molecules dimerize. In general, the phosphate groups introduced are gamma phosphates from nucleoside triphosphates, most commonly ATP.

Sfi1 homolog, spindle assembly associated (yeast) is a protein that in humans is encoded by the SFI1 gene. It localizes to the centriole, and its S. pombe ortholog has been shown to be involved in spindle pole body duplication. SFI1 forms a complex with centrin 2.