Activation and transport into mitochondria
Fatty acids must be activated before they can be carried into the mitochondria, where fatty acid oxidation occurs. This process occurs in two steps catalyzed by the enzyme fatty acyl-CoA synthetase.
Formation of an activated thioester bond
The enzyme first catalyzes nucleophilic attack on the α-phosphate of ATP to form pyrophosphate and an acyl chain linked to AMP. The next step is formation of an activated thioester bond between the fatty acyl chain and Coenzyme A.
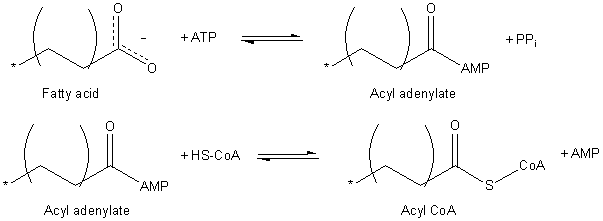
The balanced equation for the above is:
RCOO− + CoASH + ATP → RCO-SCoA + AMP + PPi
This two-step reaction is freely reversible and its equilibrium lies near 1. To drive the reaction forward, the reaction is coupled to a strongly exergonic hydrolysis reaction: the enzyme inorganic pyrophosphatase cleaves the pyrophosphate liberated from ATP to two phosphate ions, consuming one water molecule in the process. Thus the net reaction becomes:
RCOO− + CoASH + ATP → RCO-SCoA+ AMP + 2Pi
Transport into the mitochondrial matrix
The inner mitochondrial membrane is impermeable to fatty acids and a specialized carnitine carrier system operates to transport activated fatty acids from cytosol to mitochondria.
Once activated, the acyl CoA is transported into the mitochondrial matrix. This occurs via a series of similar steps:
- Acyl CoA is conjugated to carnitine by carnitine acyltransferase I (palmitoyltransferase) I located on the outer mitochondrial membrane
- Acyl carnitine is shuttled inside by a translocase
- Acyl carnitine (such as Palmitoylcarnitine) is converted to acyl CoA by carnitine acyltransferase (palmitoyltransferase) II located on the inner mitochondrial membrane. The liberated carnitine returns to the cytosol.
Carnitine acyltransferase I undergoes allosteric inhibition as a result of malonyl-CoA, an intermediate in fatty acid biosynthesis, in order to prevent futile cycling between beta-oxidation and fatty acid synthesis.
The mitochondrial oxidation of fatty acids takes place in three major steps:
- β-oxidation occurs to convert fatty acids into 2-carbon acetyl-CoA units.
- Acetyl-CoA enters into TCA cycle to yield generate reduced NADH and reduced FADH2.
- Reduced cofactors NADH and FADH2 participate in the electron transport chain in the mitochondria to yield ATP. There is no direct participation of the fatty acid.