
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.

HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
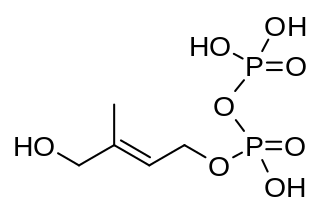
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis. The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
In enzymology, a 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase (HMB-PP synthase, IspG, EC 1.17.7.1) is an enzyme that catalyzes the chemical reaction
2-C-Methyl-D-erythritol 2,4-cyclodiphosphate synthase is a zinc-dependent enzyme and a member of the YgbB N terminal protein domain, which participates in the MEP pathway of isoprenoid precursor biosynthesis. It catalyzes the following reaction:

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction
In enzymology, a 1-deoxy-d-xylulose-5-phosphate synthase (EC 2.2.1.7) is an enzyme in the non-mevalonate pathway that catalyzes the chemical reaction

In enzymology, a 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase is an enzyme that catalyzes the chemical reaction

In enzymology, a 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase is an enzyme that catalyzes the chemical reaction:
In enzymology, a 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol kinase is an enzyme that catalyzes the chemical reaction

2-C-Methyl-d-erythritol-2,4-cyclopyrophosphate (MEcPP) is an intermediate in the MEP pathway (non-mevalonate) of isoprenoid precursor biosynthesis. MEcPP is produced by MEcPP synthase (IspF) and is a substrate for HMB-PP synthase (IspG).
The fnr gene of Escherichia coli encodes a transcriptional activator (FNR) which is required for the expression of a number of genes involved in anaerobic respiratory pathways. The FNR protein of E. coli is an oxygen – responsive transcriptional regulator required for the switch from aerobic to anaerobic metabolism.
"Type III mutants, originally frdB, were designated fnr because they were defective in fumarate and nitrate reduction and impaired in their ability to produce gas." - Lambden and Guest, 1976 Journal of General Microbiology97, 145-160

In molecular biology, the HMG-CoA reductase family is a family of enzymes which participate in the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids.

In molecular biology, YgbB is a protein domain. This entry makes reference to a number of proteins from eukaryotes and prokaryotes which share this common N-terminal signature and appear to be involved in terpenoid biosynthesis. The YgbB protein is a putative enzyme thought to aid terpenoid and isoprenoid biosynthesis, a vital chemical in all living organisms. This protein domain is part of an enzyme which catalyses a reaction in a complex pathway.

4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction
In enzymology, a 4-hydroxy-tetrahydrodipicolinate reductase (EC 1.17.1.8) is an enzyme that catalyzes the chemical reaction
Michel Rohmer, born on 31 January 1948, is a French chemist specialising in the chemistry of micro-organisms. He has particularly studied isoprenoids.













