Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol, or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Peroxidases or peroxide reductases are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides.

Chemiluminescence is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence.

Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents, but insoluble in water.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

The glucose oxidase enzyme also known as notatin is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.

In biochemistry, ABTS is a chemical compound used to observe the reaction kinetics of specific enzymes. A common use for it is in the enzyme-linked immunosorbent assay (ELISA) to detect the binding of molecules to each other.

A dot blot is a technique in molecular biology used to detect proteins. It represents a simplification of the western blot method, with the exception that the proteins to be detected are not first separated by electrophoresis. Instead, the sample is applied directly on a membrane in a single spot, and the blotting procedure is performed.

3,3′,5,5′-Tetramethylbenzidine or TMB is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). TMB is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights. Used to detect hematuria as it turns blue in contact with hemoglobin.
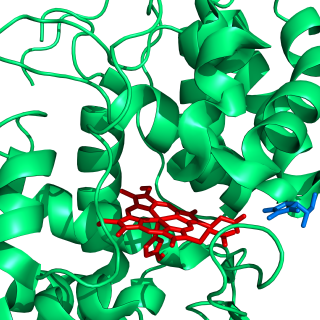
Ascorbate peroxidase (or L-ascorbate peroxidase, APX or APEX) (EC 1.11.1.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a malate oxidase (EC 1.1.3.3) is an enzyme that catalyzes the chemical reaction
Chloride peroxidase (EC 1.11.1.10) is a family of enzymes that catalyzes the chlorination of organic compounds. This enzyme combines the inorganic substrates chloride and hydrogen peroxide to produce the equivalent of Cl+, which replaces a proton in hydrocarbon substrate:
In enzymology, a lignin peroxidase (EC 1.11.1.14) is an enzyme that catalyzes the chemical reaction
In enzymology, a manganese peroxidase (EC 1.11.1.13) is an enzyme that catalyzes the chemical reaction
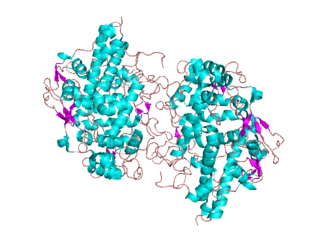
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin.
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment.
The Trinder glucose activity test is a diagnostic test used in medicine to determine the presence of glucose or glucose oxidase. The test employs the Trinder reagent, and is a colour change test resulting from the Trinder reaction.
Eclox, which stands for Enhanced ChemiLuminescence and OXyradical test for water quality analysis, is a rapid toxicity technology that has been shown to correlate with other established toxicity tests. Rapid toxicity testing is unable to identify specific contaminants or their concentrations and instead function as a screening tool to quickly determine whether water is potentially toxic.
Haem peroxidases (or heme peroxidases) are haem-containing enzymes that use hydrogen peroxide as the electron acceptor to catalyse a number of oxidative reactions. Most haem peroxidases follow the reaction scheme:
Chemiluminescence is the emission of light through a chemical reaction. It contrasts with fluorescence, which is excited by a light source. During chemiluminescence, the vibrationally excited product of an exoergic chemical reaction relaxes to its ground state with the emission of photons. Since the process does not require excitation light, problems in its application caused by light scattering or source instability are absent, and there is no concern about autofluorescence in the background, which can lead to highly sensitive deep tissue imaging.











