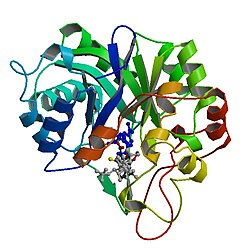
Alcohol dehydrogenase [NADP+] also known as aldehyde reductase or aldo-keto reductase family 1 member A1 is an enzyme that in humans is encoded by the AKR1A1 gene. [5] [6] [7] AKR1A1 belongs to the aldo-keto reductase (AKR) superfamily. It catalyzes the NADPH-dependent reduction of a variety of aromatic and aliphatic aldehydes to their corresponding alcohols and catalyzes the reduction of mevaldate to mevalonic acid and of glyceraldehyde to glycerol. [8] Mutations in the AKR1A1 gene has been found associated with non-Hodgkin's lymphoma. [9]