Related Research Articles
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.

Sialyltransferases are enzymes that transfer sialic acid to nascent oligosaccharide. Each sialyltransferase is specific for a particular sugar substrate. Sialyltransferases add sialic acid to the terminal portions of the sialylated glycolipids (gangliosides) or to the N- or O-linked sugar chains of glycoproteins.
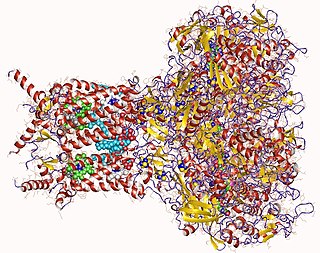
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase (EC 1.17.1.9) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming.
D-amino-acid dehydrogenase is a bacterial enzyme that catalyses the oxidation of D-amino acids into their corresponding oxoacids. It contains both flavin and nonheme iron as cofactors. The enzyme has a very broad specificity and can act on most D-amino acids.
In enzymology, a D-arabinose 1-dehydrogenase (EC 1.1.1.116) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-oxo-5beta-steroid 4-dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a meso-tartrate dehydrogenase (EC 1.3.1.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a choline dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a dehydrogluconate dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a gluconate 2-dehydrogenase (acceptor) is an enzyme that catalyzes the chemical reaction
In enzymology, a malate dehydrogenase (quinone) (EC 1.1.5.4), formerly malate dehydrogenase (acceptor) (EC 1.1.99.16), is an enzyme that catalyzes the chemical reaction
In enzymology, a pyridoxine 5-dehydrogenase is an enzyme that catalyzes the chemical reaction
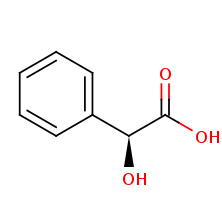
In enzymology, (S)-mandelate dehydrogenase (MDH), is an enzyme that catalyzes the chemical reaction.
In enzymology, a cytokinin dehydrogenase (EC 1.5.99.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycine dehydrogenase (EC 1.4.1.10) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADPH dehydrogenase (EC 1.6.99.1) is an enzyme that catalyzes the chemical reaction
Pyranose dehydrogenase (acceptor) (EC 1.1.99.29, pyranose dehydrogenase, pyranose-quinone oxidoreductase, quinone-dependent pyranose dehydrogenase, PDH) is an enzyme with systematic name pyranose:acceptor oxidoreductase. This enzyme catalyses the following chemical reaction
NADH dehydrogenase is an enzyme that converts nicotinamide adenine dinucleotide (NAD) from its reduced form (NADH) to its oxidized form (NAD+). Members of the NADH dehydrogenase family and analogues are commonly systematically named using the format NADH:acceptor oxidoreductase. The chemical reaction these enzymes catalyze is generally represented with the following equation:
References
- ↑ Kulys, Juozas; Tetianec, Lidija; Bratkovskaja, Irina (2010). "Pyrroloquinoline quinone-dependent carbohydrate dehydrogenase: Activity enhancement and the role of artificial electron acceptors". Biotechnology Journal. 5 (8): 822–828. doi:10.1002/biot.201000119. PMID 20669254.