
Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis (dandruff). It may be used in combination with ultraviolet light therapy. Industrially it is a railroad tie preservative and used in the surfacing of roads. Coal tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government, issued in 1980.

Smoke is a suspension of airborne particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product of fires, but may also be used for pest control (fumigation), communication, defensive and offensive capabilities in the military, cooking, or smoking. It is used in rituals where incense, sage, or resin is burned to produce a smell for spiritual or magical purposes. It can also be a flavoring agent and preservative.

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Soot is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Soot is considered a hazardous substance with carcinogenic properties. Most broadly, the term includes all the particulate matter produced by this process, including black carbon and residual pyrolysed fuel particles such as coal, cenospheres, charred wood, and petroleum coke classified as cokes or char. It can include polycyclic aromatic hydrocarbons and heavy metals like mercury.

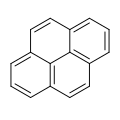
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, explosives, and drugs. It has also been used to make bile acids, cholesterol and steroids.

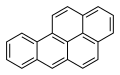
Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. The chemical formula is C16H10. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure. They are especially useful in quantifying an organism's exposure to a carcinogen. The presence of such an adduct indicates prior exposure to a potential carcinogen, but it does not necessarily indicate the presence of cancer in the subject animal.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

A benzopyrene is an organic compound with the formula C20H12. Structurally speaking, the colorless isomers of benzopyrene are pentacyclic hydrocarbons and are fusion products of pyrene and a phenylene group. Two isomeric species of benzopyrene are benzo[a]pyrene and the less common benzo[e]pyrene. They belong to the chemical class of polycyclic aromatic hydrocarbons.

Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is produced during incomplete combustion of organic matter.
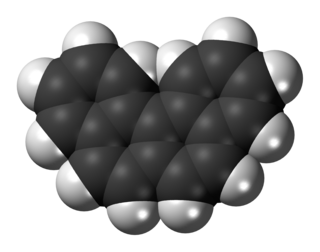
Benzo[c]phenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
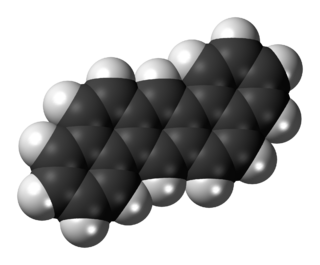
Dibenz[a,h]anthracene or Benzo[k]tetraphene or 1,2:5,6-Dibenzanthracene is an organic compound with the chemical formula C22H14. It is a polycyclic aromatic hydrocarbon (PAH) made of five fused benzene rings. It is a fused five-ringed PAH which is common as a pollutant of smoke and oils. It is stable and highly genotoxic in bacterial and mammalian cell systems, as it intercalates into DNA and causes mutations.
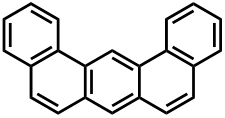
Dibenz[a,j]anthracene or Benzo[m]tetraphene or 1,2:7,8-Dibenzanthracene is an organic compound with the chemical formula C22H14. It belongs to the class of polycyclic aromatic hydrocarbons (PAHs) and is formed whenever there is incomplete combustion of organic matter. The IARC (International Agency for Research on Cancer) has classified it as possibly carcinogenic to humans, grouped into IARC group 2B.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
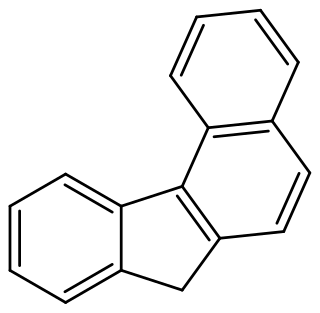
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.

Indeno(1,2,3-cd)pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples, including indeno(1,2,3-cd)pyrene (IP). In 1962, the National Cancer Institute reported that indeno(1,2,3-cd)pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.




















![An adduct formed between a DNA strand and an epoxide derived from a benzo[a]pyrene molecule (center); such adducts may interfere with normal DNA replication. Benzopyrene DNA adduct 1JDG.png](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d8/Benzopyrene_DNA_adduct_1JDG.png/150px-Benzopyrene_DNA_adduct_1JDG.png)



















