Aromatic compounds are organic compounds are "mono- and polycyclic aromatic hydrocarbons". The parent member is benzene. Heteroarenes are closely related by at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom.

In chemistry, a conjugated system is a system of connected p orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

In chemistry, aromaticity is a property of cyclic (ring-shaped), typically planar (flat) molecular structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or ions by the combination of several contributing structures into a resonance hybrid in valence bond theory. It has particular value for describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
A carbanion is an anion in which carbon is trivalent and bears a formal negative charge.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.
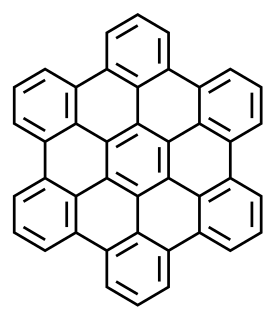
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised electrons in it. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.

Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula C8H6. It is antiaromatic, because it has 4n π electrons where n is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-tert-butylpentalene was synthesized in 1973. Because of the tert-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene.

In chemistry, the organic compound triphenylene is a flat polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings. Triphenylene can be isolated from coal tar. It is also made synthetically by synthesis and trimerization of benzyne. One molecule of triphenylene has delocalized 18-π-electron systems based on a planar structure. It has the molecular formula C
18H
12.
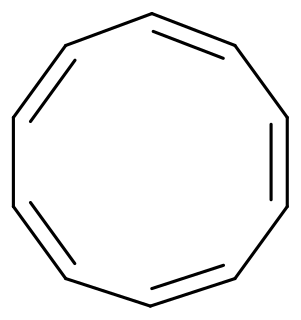
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps, the opposite pattern compared to the aromatic character to Hückel systems. The nodal plane of the orbitals, viewed as a ribbon, is a Möbius strip, rather than a cylinder, hence the name. The pattern of orbital energies is given by a rotated Frost circle (with the edge of the polygon on the bottom instead of a vertex), so systems with 4n electrons are aromatic, while those with 4n + 2 electrons are anti-aromatic/non-aromatic. Due to incrementally twisted nature of the orbitals of a Möbius aromatic system, stable Möbius aromatic molecules need to contain at least 8 electrons, although 4 electron Möbius aromatic transition states are well known in the context of the Dewar-Zimmerman framework for pericyclic reactions. Möbius molecular systems were considered in 1964 by Edgar Heilbronner by application of the Hückel method, but the first such isolable compound was not synthesized until 2003 by the group of Rainer Herges. However, the fleeting trans-C9H9+ cation, one conformation of which is shown on the right, was proposed to be a Möbius aromatic reactive intermediate in 1998 based on computational and experimental evidence.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Sesquifulvalene or Pentaheptafulvalene is a hydrocarbon in the fulvalene class with chemical formula C12H10. It is composed of linked cyclopentadiene and cycloheptatriene rings.

Calicene or triapentafulvalene is a hydrocarbon of the fulvalene class with chemical formula C8H6, composed of a cyclopentadiene ring and a cyclopropene ring linked by a double bond. Its name is derived from the Latin calix meaning "goblet", from its shape.
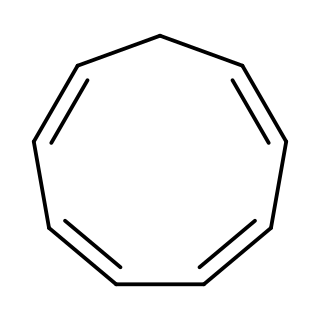
Cyclononatetraene is an organic compound with the formula C9H10. It was first prepared in 1969 by protonation of the corresponding aromatic anion (described below). It is unstable and isomerizes with a half-life of 50 minutes at room temperature to 3a,7a-dihydro-1H-indene via a thermal 6π disrotatory electrocyclic ring closing. Upon exposure to ultraviolet light, it undergoes a photochemical 8π electrocyclic ring closing to give bicyclo[6.1.0]nona-2,4,6-triene.

Butalene is a polycyclic hydrocarbon composed of two fused cyclobutadiene rings. A reported possible synthesis of it involves an elimination reaction from a Dewar benzene derivative. The structure itself can be envisioned as benzene with an internal bridge, and calculations indicate it is somewhat less stable than the open 1,4-didehydrobenzene biradical, the valence isomer in which that bridged bond is broken.

















