 | |
 | |
 | |
| Names | |
|---|---|
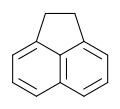
| Preferred IUPAC name 1,2-Dihydroacenaphthylene | |
| Other names 1,8-Ethylenenaphthalene peri-Ethylenenaphthalene Naphthyleneethylene Tricyclo[6.3.1.04,12]dodecapentaene Tricyclo[6.3.1.04,12]dodeca-1(12),4,6,8,10-pentaene | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.336 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C12H10 | |
| Molar mass | 154.212 g·mol−1 |
| Appearance | White or pale yellow crystalline powder |
| Density | 1.024 g/cm3 |
| Melting point | 93.4 °C (200.1 °F; 366.5 K) |
| Boiling point | 279 °C (534 °F; 552 K) |
| 0.4 mg/100 ml | |
| Solubility in ethanol | slight |
| Solubility in chloroform | slight |
| Solubility in benzene | very soluble |
| Solubility in acetic acid | soluble |
| Vapor pressure | 0.001 to 0.01 mmHg at 68°F; 5 mmHg at 238.6°F [1] |
| −0.709·10−6 cm3/g | |
| Thermochemistry [2] | |
Heat capacity (C) | 190.4 J mol−1 K−1 |
Std molar entropy (S⦵298) | 188.9 J mol−1 K−1 |
Std enthalpy of formation (ΔfH⦵298) | 70.3 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 135 °C (275 °F; 408 K) |
| >450 °C (842 °F; 723 K) | |
| Safety data sheet (SDS) | ICSC 1674 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound. [3]

