
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell.

In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), typically planar (flat) molecular structures with pi bonds in resonance that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term aromaticity with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds.

In chemistry, pi stacking refers to the presumptive attractive, noncovalent pi interactions between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings is electrostatically repulsive. What is more commonly observed is either a staggered stacking or pi-teeing interaction both of which are electrostatic attractive For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties. Fluorene is mildly acidic.

An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.

A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids. Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include [5]circulene (corannulene), [6]circulene (coronene), [7]circulene, and [12]circulene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure. The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.
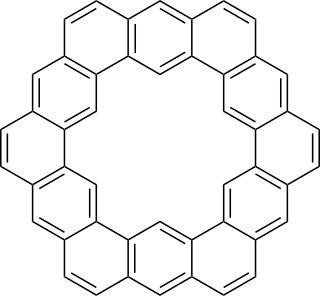
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a [12]-circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929 Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
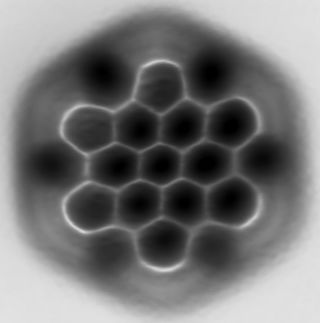
Hexa-peri-hexabenzocoronene (HBC) is a polycyclic aromatic hydrocarbon with the molecular formula C42H18. It consists of a central coronene molecule, with an additional benzene ring fused between each adjacent pair of rings around the periphery. It is sometimes simply called hexabenzocoronene, however, there are other chemicals that share this less-specific name, such as hexa-cata-hexabenzocoronene.

Contorted aromatics or more precisely contorted polycyclic aromatic hydrocarbons are polycyclic aromatic hydrocarbons (PAHs) in which the fused aromatic molecules deviate from the usual planarity.

Klaus Müllen is a German chemist working in the fields of polymer chemistry, supramolecular chemistry and nanotechnology. He is known for the synthesis and exploration of the properties of graphene-like nanostructures and their potential applications in organic electronics.
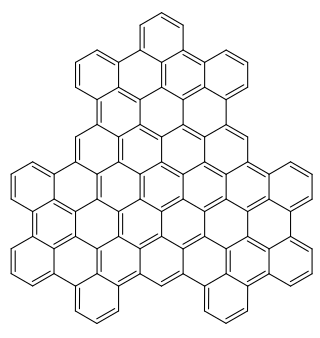
Superphenalene is a very large polycyclic aromatic hydrocarbon (PAH) with chemical formula C96H30. It can be formally considered to consist of three fused superbenzenes (hexa-peri-hexabenzocoronene).

















