In chemistry, aromaticity means a molecule has a cyclic (ring-shaped) structure with pi bonds in resonance. Aromatic rings give increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term aromaticity with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.

Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds ('mancude'). They have the general formula CnHn (when n is an even number) or CnHn+1 (when n is an odd number). The IUPAC accepts the use of 'annulene nomenclature' in naming carbocyclic ring systems with 7 or more carbon atoms, using the name '[n]annulene' for the mancude hydrocarbon with n carbon atoms in its ring, though in certain contexts (e.g., discussions of aromaticity for different ring sizes), smaller rings (n = 3 to 6) can also be informally referred to as annulenes. Using this form of nomenclature 1,3,5,7-cyclooctatetraene is [8]annulene and benzene is [6]annulene (and occasionally referred to as just 'annulene').
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
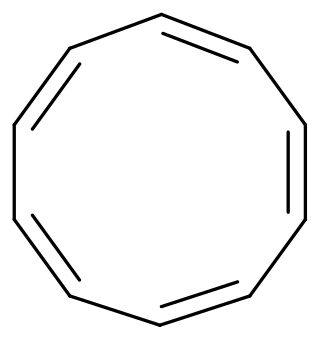
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry.
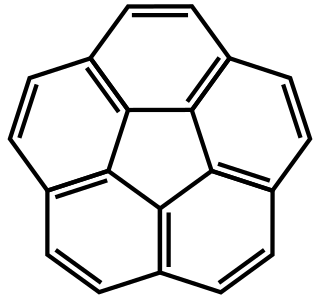
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.

Cyclooctadecanonaene or [18]annulene is an organic compound with chemical formula C
18H
18. It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that [18]annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis,trans,trans,cis,trans,trans,cis,trans,trans configuration. It is reported to be a red-brown crystalline solid.
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Annulynes or dehydroannulenes are conjugated monocyclic hydrocarbons with alternating single and double bonds in addition to at least one triple bond.
Franz Sondheimer FRS was a German-born British professor of chemistry. In 1960, he was awarded the Israel Prize for his contributions to science.

Hexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility.

Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings. This compound has long been pursued by chemists because of its potential interest in electronic applications and was first synthesized but not cleanly isolated in 2006. Heptacene was finally fully characterized in bulk by researchers in Germany and the United States in 2017.
In organic and physical organic chemistry, Clar's rule is an empirical rule that relates the chemical stability of a molecule with its aromaticity. It was introduced in 1972 by the Austrian organic chemist Erich Clar in his book The Aromatic Sextet. The rule states that given a polycyclic aromatic hydrocarbon, the resonance structure most important to characterize its properties is that with the largest number of aromatic π-sextets i.e. benzene-like moieties.

1H-Phenalene, often called simply phenalene is a polycyclic aromatic hydrocarbon (PAH). Like many PAHs, it is an atmospheric pollutant formed during the combustion of fossil fuels. It is the parent compound for the phosphorus-containing phosphaphenalenes.
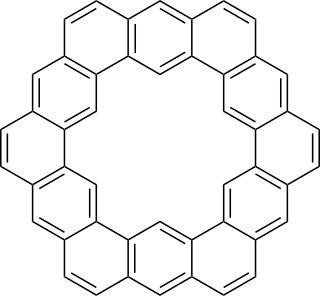
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a [12]-circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
Cyclotetradecaheptaene, often referred to as [14]annulene, is a hydrocarbon with molecular formula C14H14, which played an important role in the development of criteria (Hückel's rule) for aromaticity, a stabilizing property of central importance in physical organic chemistry. It forms dark-red needle-like crystals.

Dibenzopentalene (dibenzo[a,e]pentalene or dibenzo[b,f]pentalene) is an organic compound and a hydrocarbon with formula C16H10. It is of some scientific interest as a stable derivative of the highly reactive antiaromatic pentalene by benzannulation. The first derivative was synthesised in 1912 by Brand. The parent compound was reported in 1952. The NICS value for the 5-membered rings is estimated at 7.4 ppm and that of the six-membered rings -9.8 ppm. Aromatic dicationic salts can be obtained by reaction with antimony pentafluoride in sulfuryl chloride. The dianion forms by reduction with lithium metal or deprotonation of 5,10-dihydroindeno[2,1-a]indene with two equivalents of butyllithium. The aromatic nature of the dianion has been confirmed by X-ray analysis. Another isomer of this compound exists called dibenzo[a,f]pentalene with one of the benzene rings positioned on the other available pentalene face.
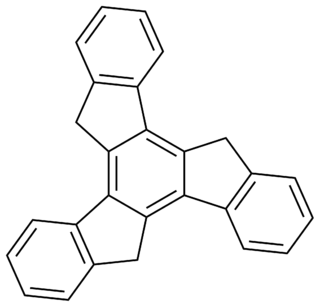
Truxene is a polycyclic aromatic hydrocarbon. The molecule can be thought of as being made up of three fluorene units arranged symmetrically and sharing a common central benzene. Truxene is solid, and it is slightly soluble in water.