
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines, at one time acridine dyes were popular but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is a key intermediate in several metabolic pathways throughout the cell.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
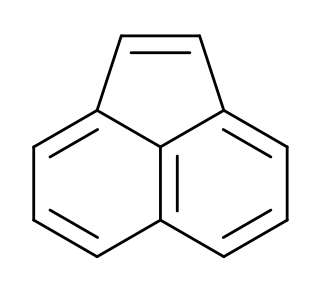
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.
Petroleum ether is the petroleum fraction consisting of aliphatic hydrocarbons and boiling in the range 35‒60 °C, and commonly used as a laboratory solvent. Despite the name, petroleum ether is not classified as an ether; the term is used only figuratively, signifying extreme lightness and volatility.
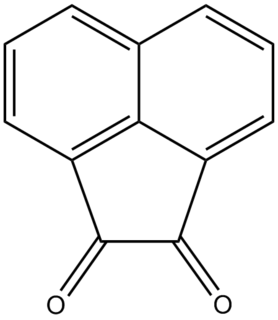
Acenaphthoquinone is a quinone derived from acenaphthene. It is a water-insoluble yellow solid. It is a precursor to some agrichemicals and dyes.
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene. Aromatization includes the formation of heterocyclic systems.

Cumene (isopropylbenzene) is an organic compound that is based on an aromatic hydrocarbon with an aliphatic substitution. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals, primarily phenol and acetone.

Graphite intercalation compounds (GICs) are complex materials having a formula CXm where the ion Xn+ or Xn− is inserted (intercalated) between the oppositely charged carbon layers. Typically m is much less than 1. These materials are deeply colored solids that exhibit a range of electrical and redox properties of potential applications.
An organic superconductor is a synthetic organic compound that exhibits superconductivity at low temperatures.
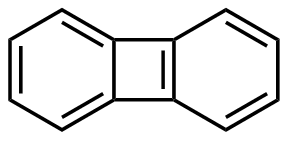
Biphenylene is an alternant, polycyclic hydrocarbon composed of two benzene rings joined together by a pair of mutual attachments, thus forming a 6-4-6 arene system. The resulting planar structure was one of the first π-electronic hydrocarbon systems discovered to show evidence of antiaromaticity.
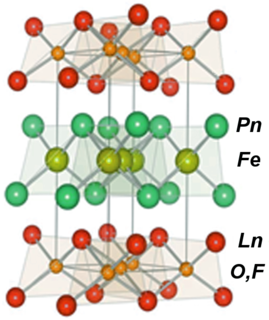
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..

Olympicene is an organic carbon based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings.

Phenacenes are a class of organic compounds consisting of fused aromatic rings. They are polycyclic aromatic hydrocarbons, related to acenes and helicenes from which they differ by the arrangement of the fused rings.
In chemistry, a C–H···O interaction is occasionally described as a special type of weak hydrogen bond. These interactions frequently occur in the structures of important biomolecules like amino acids, proteins, sugars, DNA and RNA.

Carbon peapod is a hybrid nanomaterial consisting of spheroidal fullerenes encapsulated within a carbon nanotube. It is named due to their resemblance to the seedpod of the pea plant. Since the properties of carbon peapods differ from those of nanotubes and fullerenes, the carbon peapod can be recognized as a new type of a self-assembled graphitic structure. Possible applications of nano-peapods include nanoscale lasers, single electron transistors, spin-qubit arrays for quantum computing, nanopipettes, and data storage devices thanks to the memory effects and superconductivity of nano-peapods.

Niobium diselenide or niobium(IV) selenide is a layered transition metal dichalcogenide with formula NbSe2. Niobium diselenide is a lubricant, and a superconductor at temperatures below 7.2 K that exhibit a charge density wave (CDW). NbSe2 crystallizes in several related forms, and can be mechanically exfoliated into monatomic layers, similar to other transition metal dichalcogenide monolayers. Monolayer NbSe2 exhibits very different properties from the bulk material, such as of Ising superconductivity, quantum metallic state, and strong enhancement of the CDW.
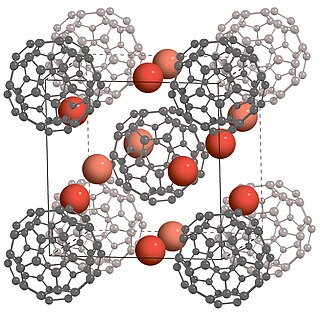
Fullerides are chemical compounds containing fullerene anions. Common fullerides are derivatives of the most common fullerenes, i.e. C60 and C70. The scope of the area is large because multiple charges are possible, i.e., [C60]n− (n = 1, 2...6), and all fullerenes can be converted to fullerides. The suffix "-ide" implies their negatively charged nature.
















