 | |
 An atomic force microscopy image of olympicene | |
 | |
| Names | |
|---|---|
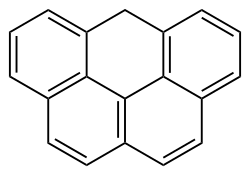
| Preferred IUPAC name 6H-Benzo[cd]pyrene | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| Properties | |
| C19H12 | |
| Molar mass | 240.305 g·mol−1 |
| Appearance | white powder |
| Density | 1.28 g/cm3 |
| Boiling point | 511.754 °C (953.157 °F; 784.904 K) at 760 mmHg |
| Hazards | |
| Flash point | 254.195 °C (489.551 °F; 527.345 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Olympicene is an organic carbon-based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings.
Contents
The molecule was conceived in March 2010 as a way to celebrate the 2012 London Olympics by Graham Richards of University of Oxford and Antony Williams. It was first synthesized by researchers Anish Mistry and David Fox of the University of Warwick in the UK. [1] [2] [3] Relative energies of olympicene and its isomers were first predicted from quantum electronic-structure computations by Andrew Valentine and David Mazziotti of the University of Chicago. [4]
