| |
 | |
| Names | |
|---|---|
| IUPAC name 6,9,12,15,18,29,32,35,38,41,52,55,58,61,64-pentadecaoxaheptacyclo[63.4.0.05,69.019,24.023,28.042,47.046,51]nonahexaconta-1(69),2,4,19,21,23,25,27,42,44,46,48,50,65,67-pentadecaene;5,12,19,26-tetrazoniaheptacyclo[24.2.2.22,5.27,10.212,15.216,19.221,24]tetraconta-1(29),2(40),3,5(39),7,9,12,14,16(34),17,19(33),21(32),22,24(31),26(30),27,35,37-octadecaene;dodecahexafluorophosphate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C228H236F72N12O30P12 | |
| Molar mass | 5364.020 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
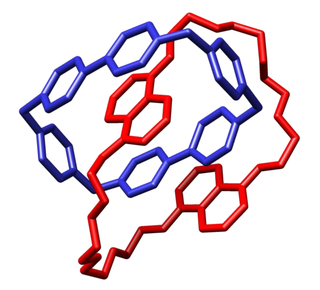
Olympiadane is a mechanically interlocked molecule composed of five interlocking macrocycles that resembles the Olympic rings. The molecule is a linear pentacatenane or a [5]catenane. It was synthesized and named by Fraser Stoddart and coworkers in 1994. [1] The molecule was designed without any practical use in mind, [2] although other catenanes may have possible application to the construction of a molecular computer.