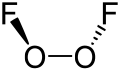This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these messages)
|
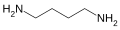
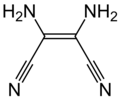
Chemical nomenclature, replete as it is with compounds with very complex names, is a repository for some names that may be considered unusual. A browse through the Physical Constants of Organic Compounds in the CRC Handbook of Chemistry and Physics (a fundamental resource) will reveal not just the whimsical work of chemists, but the sometimes peculiar compound names that occur as the consequence of simple juxtaposition. Some names derive legitimately from their chemical makeup, from the geographic region where they may be found, the plant or animal species from which they are isolated or the name of the discoverer.
Contents
- Elements
- Compounds
- Name based on shape
- Named after people
- Named after fictional characters
- Related to sex
- Related to bodily functions
- Related to death and decay
- Related to religion or legend
- Sounds like a name (person, brand or organization)
- A part sounds like an English word
- Other
- See also
- References
- Bibliography
Some are given intentionally unusual trivial names based on their structure, a notable property or at the whim of those who first isolate them. However, many trivial names predate formal naming conventions. Trivial names can also be ambiguous or carry different meanings in different industries, geographic regions and languages.
Godly noted that "Trivial names having the status of INN or ISO are carefully tailor-made for their field of use and are internationally accepted". [1] In his preface to Chemical Nomenclature, Thurlow wrote that "Chemical names do not have to be deadly serious". [2] A website in existence since 1997 [3] and maintained at the University of Bristol lists a selection of "molecules with silly or unusual names" strictly for entertainment. These so-called silly or funny trivial names (depending on culture) can also serve an educational purpose. In an article in the Journal of Chemical Education , Dennis Ryan argues that students of organic nomenclature (considered a "dry and boring" subject) may actually take an interest in it when tasked with the job of converting funny-sounding chemical trivial names to their proper systematic names. [4]
The collection listed below presents a sample of trivial names and gives an idea how chemists are inspired when they coin a brand new name for a chemical compound outside of systematic naming. It also includes some examples of systematic names and acronyms that accidentally resemble English words.








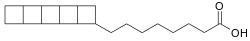







![[1.1.1]Propellane 1.1.1-propellane.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/7/79/1.1.1-propellane.svg/120px-1.1.1-propellane.svg.png)