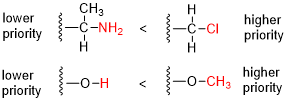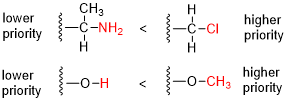
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4.

A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. Simply this is the structural unit of carbohydrates.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
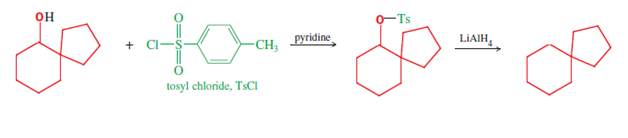
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.
Cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at 2nd position of THF with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

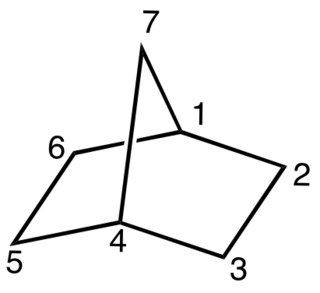
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene. Due to its unusual molecular geometry, the compound is of considerable interest to theoretical chemists.

A fenestrane in organic chemistry is a type of chemical compound with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compounds twice over. Because of their inherent strain and instability, fenestranes are of theoretical interest to chemists. The name—proposed in 1972 by Vlasios Georgian and Martin Saltzman—is derived from the Latin word for window, fenestra. Georgian had intended that "fenestrane" solely referred to [4.4.4.4]fenestrane, whose skeletal structure looks like windows, and Kenneth B. Wiberg called that specific structure "windowpane". The term fenestrane has since become generalized to refer to the whole class of molecules that have various other ring-sizes. Georgian recommended rosettane for the class, based on the structural appearance as a rosette of flowers.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
Trimethylenemethane cycloaddition is the formal [3+2] annulation of trimethylenemethane (TMM) derivatives to two-atom pi systems. Although TMM itself is too reactive and unstable to be stored, reagents which can generate TMM or TMM synthons in situ can be used to effect cycloaddition reactions with appropriate electron acceptors. Generally, electron-deficient pi bonds undergo cyclization with TMMs more easily than electron-rich pi bonds.
In chemistry, a ring is an ambiguous term referring either to a simple cycle of atoms and bonds in a molecule or to a connected set of atoms and bonds in which every atom and bond is a member of a cycle. A ring system that is a simple cycle is called a monocycle or simple ring, and one that is not a simple cycle is called a polycycle or polycyclic ring system. A simple ring contains the same number of sigma bonds as atoms, and a polycyclic ring system contains more sigma bonds than atoms.
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DVTOSU) is a bicyclic organic molecule having a central quaternary carbon atom with which two alicyclic rings are linked, each comprising five atoms. DVTOSU is a diallyl acetal and the precursor for the isomeric ketene acetal monomer 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane (DETOSU) which is a building block for polyorthoesters.
![Preparation of a heterocyclic spiro compound. Each molecule shown in line-angle representation, where each angle (vertex) represents a carbon atom, and each line a bond between atoms (and where necessary hydrogen atoms to fulfill carbon's valence of four are implied). The heterocyclic spiro product, at right of arrow, is a common ketal (acetal) structure prepared from a monocyclic type of ketone and a generally acyclic type of diol (both at left of arrow). The schematic is for the synthesis of the protected 1,4-dioxaspiro[4.5]decane from cyclohexanone and the alcohol, ethanediol. Note the apparent planarity, which masks the actual orthogonal orientation of the two rings. Ketal Synthesis V.1.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/6/69/Ketal_Synthesis_V.1.svg/405px-Ketal_Synthesis_V.1.svg.png)
![A fully carbo-cyclic spiro compound. Spiro[5.5]undecane, again shown in line-angle representation and in apparent planarity, masking the actual most populated chair conformation of each ring, and their actual orthogonal orientation to one another. Spiro 5.5 undecan.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/9/92/Spiro_5.5_undecan.svg/125px-Spiro_5.5_undecan.svg.png)



![Two examples of spiro compound nomenclature, A. 1-bromo-3-chlorospiro[4.5]decan-7-ol, and B. 1-bromo-3-chlorospiro[3.6]decan-7-ol. Spiroverbindung Nomenklatur.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Spiroverbindung_Nomenklatur.svg/210px-Spiroverbindung_Nomenklatur.svg.png)