Aromatic compounds are organic compounds also known as "mono- and polycyclic aromatic hydrocarbons". The parent member is benzene. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom.

In chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
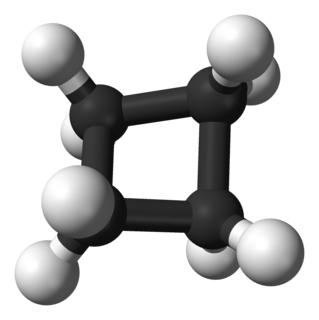
In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomer of Alkene.

Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
In chemistry, orbital hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies.
A cycloaddition is a chemical reaction in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile.

The ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond, in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule.

In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a similar "bent" structure within small ring molecules, such as cyclopropane (C3H6) or as a representation of double or triple bonds within a compound that is an alternative to the sigma and pi bond model.
Pyramidal alkenes are alkenes in which the two carbon atoms making up the double bond are not coplanar with their four substituents. This deformation results from geometric constraints. Pyramidal alkenes only are of interest because much can be learned from them about the nature of chemical bonding.

In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−1⁄3) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral.
In organic chemistry, propellane is any member of a class of polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon covalent bond. The name derives from a supposed resemblance of the molecule to a propeller: namely, the rings would be the propeller's blades, and the shared C–C bond would be its axis. The concept was introduced in 1966 by D. Ginsburg Propellanes with small cycles are highly strained and unstable, and are easily turned into polymers with interesting structures, such as staffanes. Partly for these reasons, they have been the object of much research. In the literature, the bond shared by the three cycles is usually called the "bridge"; the shared carbon atoms are then the "bridgeheads". The notation [x.y.z]propellane means the member of the family whose rings have x, y, and z carbons, not counting the two bridgeheads; or x + 2, y + 2, and z + 2 carbons, counting them. The chemical formula is therefore C
2+x+y+zH
2(x+y+z). The minimum value for x, y, and z is 1, meaning a 3-carbon ring. There is no structural ordering between the rings, so, for example, [1.3.2]propellane is the same substance as [3.2.1]propellane. Therefore, it is customary to sort the indices in decreasing order, x ≥ y ≥ z.
Trimethylenemethane cycloaddition is the formal [3+2] annulation of trimethylenemethane (TMM) derivatives to two-atom pi systems. Although TMM itself is too reactive and unstable to be stored, reagents which can generate TMM or TMM synthons in situ can be used to effect cycloaddition reactions with appropriate electron acceptors. Generally, electron-deficient pi bonds undergo cyclization with TMMs more easily than electron-rich pi bonds.
In organic chemistry, enone–alkene cycloadditions are a version of the [2+2] cycloaddition This reaction involves an enone and alkene as substrates. Although the concerted photochemical [2+2] cycloaddition is allowed, the reaction between enones and alkenes is stepwise and involves discrete diradical intermediates.

In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.
Cyclopentyne is a cycloalkyne containing five carbon atoms in the ring. Due to the ideal bond angle of 180° at each atom of the alkyne but the structural requirement that the bonds form a ring, this chemical is a highly strained structure, and the triple bond is highly reactive. The triple bond easily undergoes both [2+2] and [4+2] cycloaddition reactions. Unlike benzyne, which undergoes a [2+2] addition with loss of stereochemistry at the alkene partner, cyclopentyne reacts with alkenes with retention of geometry of the partner, an example of the relevance of orbital symmetry even for highly reactive structures. The structure can also form a π complex with lithium cations, which affects the cycloaddition reactivity. It can even interact strongly enough with copper species to form a novel type of metallacycle.
![Fenestranes, from left to right: generic fenestrane, [4.4.4.4]fenestrane with carbon atoms shown and [5.5.5.5]fenestrane Fenestranes.png](http://upload.wikimedia.org/wikipedia/commons/3/39/Fenestranes.png)

![[3.3.3.3]Fenestrane is the smallest possible structure. See the SS Pyramidanes section for discussion of its geometry. 3.3.3.3-fenestrane.png](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ec/3.3.3.3-fenestrane.png/165px-3.3.3.3-fenestrane.png)
![c,t,c,c-[4.5.5.5]Fenestrane, with ring-sizes and the one trans ring-fusion highlighted. C,t,c,c-4.5.5.5-fenestrane.png](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0e/C%2Ct%2Cc%2Cc-4.5.5.5-fenestrane.png/165px-C%2Ct%2Cc%2Cc-4.5.5.5-fenestrane.png)











