| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Cyclohex-2-en-1-one | |||
| Other names 1-Cyclohex-2-enone | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1280477 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.012.021 | ||
| EC Number |
| ||
| 2792 | |||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C6H8O | |||
| Molar mass | 96.129 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
| Density | 0.993 g/mL [1] | ||
| Melting point | −53 °C (−63 °F; 220 K) [1] | ||
| Boiling point | 171 to 173 °C (340 to 343 °F; 444 to 446 K) [1] | ||
| 41.3 g/L (25 °C) | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H226, H301, H310, H319, H330, H331 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P270, P271, P280, P284, P301+P310, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P320, P321, P322, P330, P337+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 220 mg kg−1 (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances. [2] It is colorless liquid, but commercial samples are often yellow.
Contents
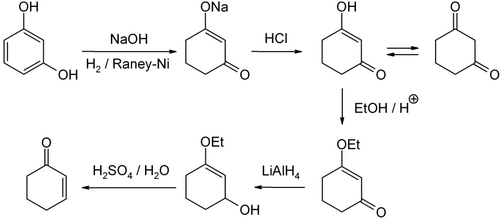
Industrially, cyclohexenone is prepared from phenol by Birch reduction. [3]
Cyclohexenone is a ketone, or more precisely an enone. Common reactions include nucleophilic conjugate addition with organocopper reagents, Michael reactions and Robinson annulations. [4] [5]