| |||
| Names | |||
|---|---|---|---|
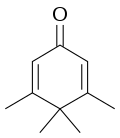
| Preferred IUPAC name 3,4,4,5-Tetramethylcyclohexa-2,5-dien-1-one | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C10H14O | |||
| Molar mass | 150.221 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Penguinone is an organic compound with the molecular formula C
10H
14O. Its name comes from the fact that its 2-dimensional molecular structure resembles a penguin. [1] [2]
The suffix "-one" indicates that it is a ketone. [3] The systematic name of the molecule is 3,4,4,5-tetramethylcyclohexa-2,5-dienone. [4] [5]
Although it is a dienone and thus has the necessary structure for a dienone–phenol rearrangement, the methyl groups in positions 3 and 5 of the ring block the movement of the group at position 4, so even the action of trifluoroacetic acid will not cause transformation to a phenol. [6]