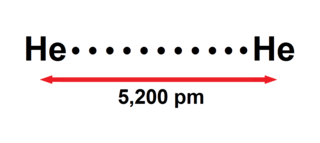A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors.
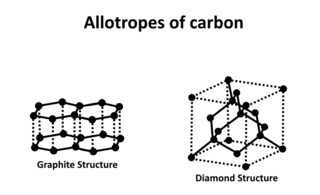
Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
In chemistry, a superatom is any cluster of atoms that seem to exhibit some of the properties of elemental atoms.

Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist: endohedral metallofullerenes and non-metal doped fullerenes.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

Metal–organic frameworks (MOFs) are a class of compounds consisting of metal clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally in various contexts such as ice, bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2.
Jing Li is a professor at Rutgers University. She and her team are engaged in solid-state, inorganic and inorganic-organic hybrid materials research. Her current research focuses on designing and developing new materials for applications in the field of renewable and sustainable energy.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.

C70 fullerene is the fullerene molecule consisting of 70 carbon atoms. It is a cage-like fused-ring structure which resembles a rugby ball, made of 25 hexagons and 12 pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge. A related fullerene molecule, named buckminsterfullerene (C60 fullerene), consists of 60 carbon atoms.
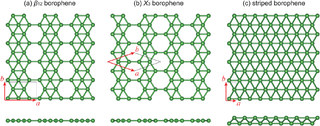
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as boron sheet. First predicted by theory in the mid-1990s, different borophene structures were experimentally confirmed in 2015.
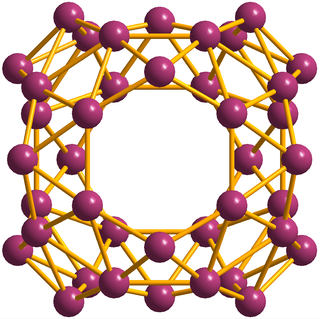
Borospherene (B40) is a cluster molecule containing 40 boron atoms. It is similar to buckminsterfullerene, the "spherical" carbon structure, but with a different symmetry. The discovery of borospherene was announced in July 2014, and is described in the journal Nature Chemistry. Borospherene is similar to other cluster molecules, including buckminsterfullerene (C60), stannaspherene, and plumbaspherene. The molecule includes unusual heptagonal faces.
A metallocarbohedryne is any one of a family of chemical compounds with the generic molecular formula M
8C
12, where M is a transition metal such as titanium, vanadium, zirconium, niobium, hafnium, molybdenum, chromium, or iron.

Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. These nanoclusters can be composed either of a single or of multiple elements, and exhibit interesting electronic, optical, and chemical properties compared to their larger counterparts.
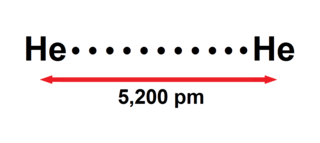
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
Carboxylate–based metal–organic frameworks are metal–organic frameworks that are based on organic molecules comprising carboxylate functional groups.