Related Research Articles

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
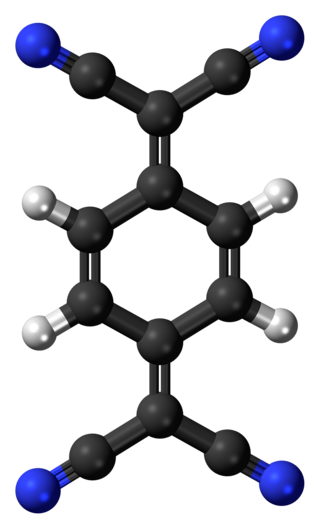
Tetracyanoquinodimethane (TCNQ) is an organic compound with the chemical formula (N≡C−)2C=C6H4=C(−C≡N)2. It is an orange crystalline solid. This cyanocarbon, a relative of para-quinone, is an electron acceptor that is used to prepare charge transfer salts, which are of interest in molecular electronics.
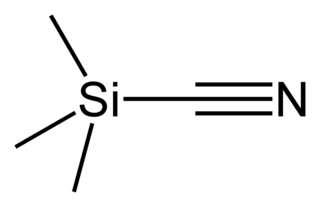
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:

Zinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.

Tetracyanoethylene (TCNE) is organic compound with the formula C2(CN)4. It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.
Electron-rich is jargon that is used in multiple related meanings with either or both kinetic and thermodynamic implications:
References
- 1 2 Webster, Owen W. "Cyanocarbons: a classic example of discovery-driven research" Journal of Polymer Science, Part A: Polymer Chemistry 2001, volume, 40, pp. 210-221. doi : 10.1002/pola.10087
- ↑ Linn, W. J. (1969). "Tetracyanoethylene Oxide". Organic Syntheses . 49: 103. doi:10.15227/orgsyn.049.0103 .
- ↑ Middleton, W. J.; Wiley, D. W. (1961). "Tetramethylammonium 1,1,2,3,3-Pentacyanopropenide". Org. Synth. 41: 99. doi:10.15227/orgsyn.041.0099.