In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.
Cyclohexene is a hydrocarbon with the formula (CH2)4C2H2. It is an example of a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an intermediate in the commercial synthesis of nylon.
Thiazole, or 1,3-thiazole, is a 5-membered heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
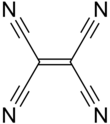
In organic chemistry, cyanocarbons are a group of chemical compounds that contain several cyanide functional groups. Such substances generally are classified as organic compounds, since they are formally derived from hydrocarbons by replacing one or more hydrogen atoms with a cyanide group. One of the simplest member is C(CN)4. Organic chemists often refer to cyanides as nitriles.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
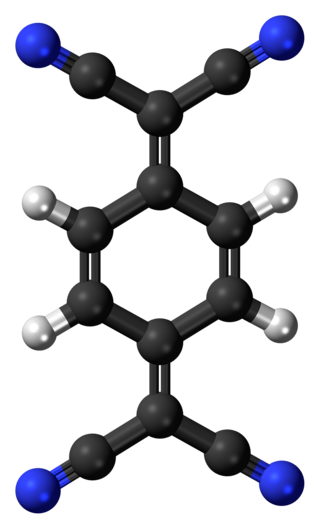
Tetracyanoquinodimethane (TCNQ) is an organic compound with the chemical formula (N≡C−)2C=C6H4=C(−C≡N)2. It is an orange crystalline solid. This cyanocarbon, a relative of para-quinone, is an electron acceptor that is used to prepare charge transfer salts, which are of interest in molecular electronics.
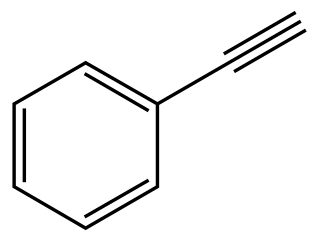
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
1-Naphthol, or α-naphthol, is an organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon (PTFE).
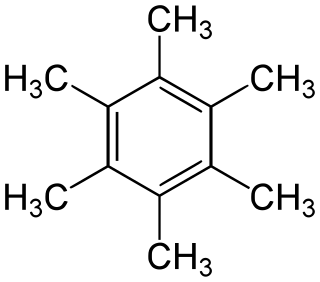
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows, one example being Pigment Yellow 74.

Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.