In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
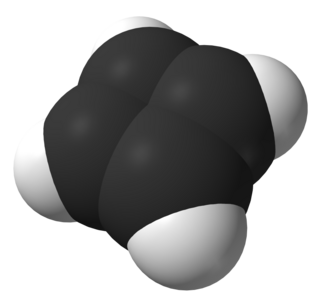
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.

Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds ('mancude'). They have the general formula CnHn (when n is an even number) or CnHn+1 (when n is an odd number). The IUPAC accepts the use of 'annulene nomenclature' in naming carbocyclic ring systems with 7 or more carbon atoms, using the name '[n]annulene' for the mancude hydrocarbon with n carbon atoms in its ring, though in certain contexts (e.g., discussions of aromaticity for different ring sizes), smaller rings (n = 3 to 6) can also be informally referred to as annulenes. Using this form of nomenclature 1,3,5,7-cyclooctatetraene is [8]annulene and benzene is [6]annulene (and occasionally referred to as just 'annulene').

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π-electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.

Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula C8H6. It is antiaromatic, because it has 4n π electrons where n is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-tert-butylpentalene was synthesized in 1973. Because of the tert-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
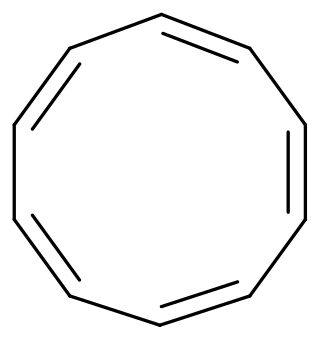
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry.

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoboranes such as B(CH3)3.

An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Cation–π interaction is a noncovalent molecular interaction between the face of an electron-rich π system (e.g. benzene, ethylene, acetylene) and an adjacent cation (e.g. Li+, Na+). This interaction is an example of noncovalent bonding between a monopole (cation) and a quadrupole (π system). Bonding energies are significant, with solution-phase values falling within the same order of magnitude as hydrogen bonds and salt bridges. Similar to these other non-covalent bonds, cation–π interactions play an important role in nature, particularly in protein structure, molecular recognition and enzyme catalysis. The effect has also been observed and put to use in synthetic systems.

In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps, the opposite pattern compared to the aromatic character to Hückel systems. The nodal plane of the orbitals, viewed as a ribbon, is a Möbius strip, rather than a cylinder, hence the name. The pattern of orbital energies is given by a rotated Frost circle (with the edge of the polygon on the bottom instead of a vertex), so systems with 4n electrons are aromatic, while those with 4n + 2 electrons are anti-aromatic/non-aromatic. Due to incrementally twisted nature of the orbitals of a Möbius aromatic system, stable Möbius aromatic molecules need to contain at least 8 electrons, although 4 electron Möbius aromatic transition states are well known in the context of the Dewar-Zimmerman framework for pericyclic reactions. Möbius molecular systems were considered in 1964 by Edgar Heilbronner by application of the Hückel method, but the first such isolable compound was not synthesized until 2003 by the group of Rainer Herges. However, the fleeting trans-C9H9+ cation, one conformation of which is shown on the right, was proposed to be a Möbius aromatic reactive intermediate in 1998 based on computational and experimental evidence.
In organic and physical organic chemistry, Clar's rule is an empirical rule that relates the chemical stability of a molecule with its aromaticity. It was introduced in 1972 by the Austrian organic chemist Erich Clar in his book The Aromatic Sextet. The rule states that given a polycyclic aromatic hydrocarbon, the resonance structure most important to characterize its properties is that with the largest number of aromatic π-sextets i.e. benzene-like moieties.

Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom respectively. They are isoelectronic with the cyclopentadienyl cation C5H+5 or abbreviated as Cp+ and comprise four π electrons. Although Hückel's rule cannot be strictly applied to borole, it is considered to be antiaromatic due to having 4 π electrons. As a result, boroles exhibit unique electronic properties not found in other metalloles.

Bicyclo[6.2.0]decapentaene is a bicyclic organic compound and an isomer of naphthalene and azulene.

Hexaphosphabenzene is a valence isoelectronic analogue of benzene and is expected to have a similar planar structure due to resonance stabilization and its sp2 nature. Although several other allotropes of phosphorus are stable, no evidence for the existence of P6 has been reported. Preliminary ab initio calculations on the trimerisation of P2 leading to the formation of the cyclic P6 were performed, and it was predicted that hexaphosphabenzene would decompose to free P2 with an energy barrier of 13−15.4 kcal mol−1, and would therefore not be observed in the uncomplexed state under normal experimental conditions. The presence of an added solvent, such as ethanol, might lead to the formation of intermolecular hydrogen bonds which may block the destabilizing interaction between phosphorus lone pairs and consequently stabilize P6. The moderate barrier suggests that hexaphosphabenzene could be synthesized from a [2+2+2] cycloaddition of three P2 molecules. Currently, this is a synthetic endeavour which remains to be conquered.