
In chemistry, a conjugated system is a system of connected p orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

In chemistry, aromaticity is a property of cyclic (ring-shaped), typically planar (flat) molecular structures with pi bonds in resonance that gives increased stability compared with other geometric or connective arrangements of the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
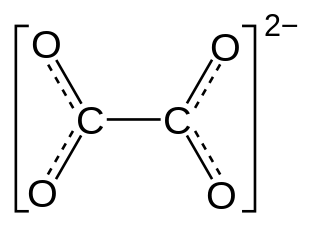
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.

Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds. They have the general formula CnHn or CnHn+1. The IUPAC naming conventions are that annulenes with 7 or more carbon atoms are named as [n]annulene, where n is the number of carbon atoms in their ring, though sometimes the smaller annulenes are referred to using the same notation, and benzene is sometimes referred to simply as annulene.
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised electrons in it. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.

Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula C8H6. It is antiaromatic, because it has 4n π electrons where n is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-tert-butylpentalene was synthesized in 1973. Because of the tert-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
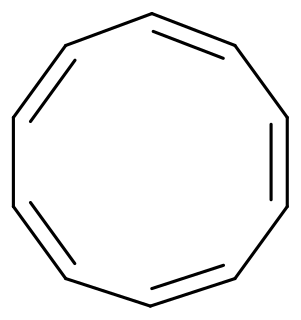
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.
Metal aromaticity or metalloaromaticity is the concept of aromaticity, found in many organic compounds, extended to metals and metal-containing compounds. The first experimental evidence for the existence of aromaticity in metals was found in aluminium cluster compounds of the type MAl−
4 where M stands for lithium, sodium or copper. These anions can be generated in a helium gas by laser vaporization of an aluminium / lithium carbonate composite or a copper or sodium / aluminium alloy, separated and selected by mass spectrometry and analyzed by photoelectron spectroscopy. The evidence for aromaticity in these compounds is based on several considerations. Computational chemistry shows that these aluminium clusters consist of a tetranuclear Al2−
4 plane and a counterion at the apex of a square pyramid. The Al2−
4 unit is perfectly planar and is not perturbed the presence of the counterion or even the presence of two counterions in the neutral compound M
2Al
4. In addition its HOMO is calculated to be a doubly occupied delocalized pi system making it obey Hückel's rule. Finally a match exists between the calculated values and the experimental photoelectron values for the energy required to remove the first 4 valence electrons. The first fully metal aromatic compound was a cyclogallane with a Ga32- core discovered by Gregory Robinson in 1995.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.

Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Cyclododecahexaene or [12]annulene is a member of the series of annulenes with some interest in organic chemistry with regard to the study of aromaticity. Cyclododecahexaene is non-aromatic due to the lack of planarity of the structure. On the other hand the dianion with 14 electrons is a Hückel aromat and more stable.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.

Croconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.
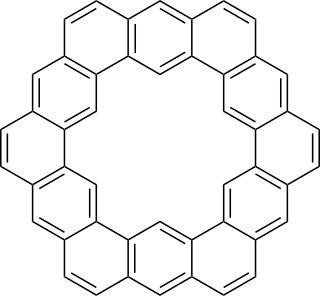
Kekulene is a polycyclic aromatic hydrocarbon and a circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.

In chemistry, the cyclooctatetraenide anion or cyclooctatetraenide, more precisely cyclooctatetraenediide, is an aromatic species with a formula of [C8H8]2− and abbreviated as COT2−. It is the dianion of cyclooctatetraene. Salts of the cyclooctatetraenide anion can be stable, e.g., Dipotassium cyclooctatetraenide or disodium cyclooctatetraenide. More complex coordination compounds are known as cyclooctatetraenide complexes, such as the actinocenes.

Bicyclo[6.2.0]decapentaene is a bicyclic organic compound and an isomer of naphthalene and azulene.















