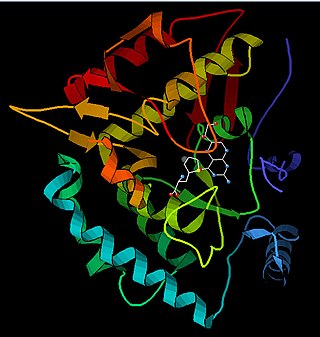
Phenylalanine hydroxylase (PAH) (EC 1.14.16.1) is an enzyme that catalyzes the hydroxylation of the aromatic side-chain of phenylalanine to generate tyrosine. PAH is one of three members of the biopterin-dependent aromatic amino acid hydroxylases, a class of monooxygenase that uses tetrahydrobiopterin (BH4, a pteridine cofactor) and a non-heme iron for catalysis. During the reaction, molecular oxygen is heterolytically cleaved with sequential incorporation of one oxygen atom into BH4 and phenylalanine substrate. In humans, mutations in its encoding gene, PAH, can lead to the metabolic disorder phenylketonuria.

An excimer is a short-lived polyatomic molecule formed from two species that do not form a stable molecule in the ground state. In this case, formation of molecules is possible only if such atom is in an electronic excited state. Heteronuclear molecules and molecules that have more than two species are also called exciplex molecules. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is very short, on the order of nanoseconds.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.

Ovalene is a polycyclic aromatic hydrocarbon with the formula C32H14, which consists of ten peri-fused six-membered rings. It is very similar to coronene.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.

Pomatoschistus is a genus of gobies native to fresh, brackish and marine waters of Europe, the eastern Atlantic Ocean and the Mediterranean Sea.
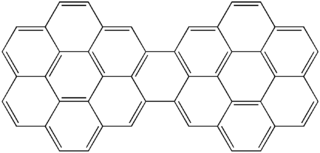
Dicoronylene is the trivial name for a very large polycyclic aromatic hydrocarbon. It has 15 rings and is a brick-red solid. Its formula is C
48H
20. Dicoronylene sublimes under high vacuum, 0.001 torr, between 250 °C and 300 °C.

In membrane biology, fusion is the process by which two initially distinct lipid bilayers merge their hydrophobic cores, resulting in one interconnected structure. If this fusion proceeds completely through both leaflets of both bilayers, an aqueous bridge is formed and the internal contents of the two structures can mix. Alternatively, if only one leaflet from each bilayer is involved in the fusion process, the bilayers are said to be hemifused. In hemifusion, the lipid constituents of the outer leaflet of the two bilayers can mix, but the inner leaflets remain distinct. The aqueous contents enclosed by each bilayer also remain separated.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.
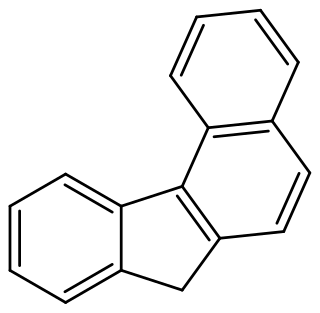
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
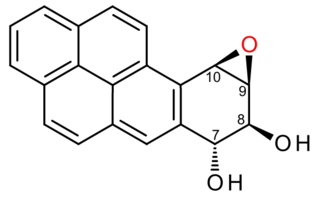
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.

Indeno[1,2,3-cd]pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples. One of these 16 is Indeno[1,2,3-cd]pyrene (IP). IP is the combination of an indeno molecule and a pyrene molecule with a fluoranthene network. In 1962, the National Cancer Institute reported that indeno[1,2,3-cd]pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.

An N-heterocyclic carbene boryl anion is an isoelectronic structure of an N-heterocyclic carbene (NHC), where the carbene carbon is replaced with a boron atom that has a -1 charge. NHC boryl anions have a planar geometry, and the boron atom is considered to be sp2-hybridized. They serve as extremely strong bases, as they are very nucleophilic. They also have a very strong trans influence, due to the σ-donation coming from the boron atom. NHC boryl anions have stronger electron-releasing character when compared to normal NHCs. These characteristics make NHC boryl anions key ligands in many applications, such as polycyclic aromatic hydrocarbons, and more commonly low oxidation state main group element bonding.
Ellipsomyxa is a genus of cnidarian that is part of the family Ceratomyxidae.




























