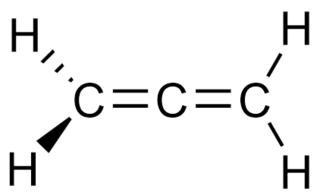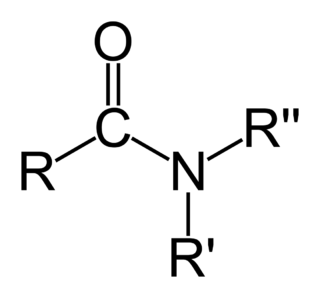
In organic chemistry, an amide ( or or, also known as an organic amide or a carboxamide, is a compound with the general formula RC NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid RC OH with the hydroxyl group –OH replaced by an amine group −NR′R″; or, equivalently, an acyl group RC − joined to an amine group.
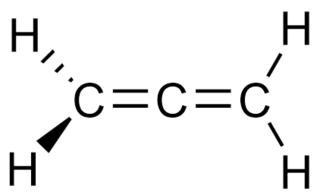
Allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

A disaccharide is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.

In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen bonds. Due to carbon's ability to catenate, millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds, along with a few other exceptions, are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive.
In biochemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. Persulfide usually refers to R−S−S−H compounds.
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.

Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless, pyrophoric gas with a repulsively sweet odor. Synonyms include boroethane, boron hydride, and diboron hexahydride. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Its derivatives are useful reagents.

The Grignard reaction is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms. In ethane, the orbitals are sp3-hybridized orbitals, but single bonds formed between carbon atoms with other hybridizations do occur. In fact, the carbon atoms in the single bond need not be of the same hybridization. Carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. A double bond is formed with an sp2-hybridized orbital and a p-orbital that is not involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from each atom. The use of the p-orbitals forms a pi bond.

An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen (H) or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base. The carbon atom has two additional single bonds. The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.

Dr. Peter Dennis Mitchell, FRS was a British biochemist who was awarded the 1978 Nobel Prize for Chemistry for his discovery of the chemiosmotic mechanism of ATP synthesis.
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of thousands of different cellular processes such as cellular respiration and the many other metabolic and enzymatic processes that lead to production and utilization of energy in forms such as adenosine triphosphate (ATP) molecules. That is, the goal of bioenergetics is to describe how living organisms acquire and transform energy in order to perform biological work. The study of metabolic pathways is thus essential to bioenergetics.

A cumulene is a hydrocarbon with three or more cumulative (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene, which is also called simply cumulene. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to alkynes, which makes them appealing for molecular nanotechnology. Polyynes are another kind of rigid carbon chains. Cumulenes are found in regions of outer space where hydrogen is rare. Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

A Grignard reagent or Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.
Carbon–hydrogen bond functionalization is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon–X bond. The term usually implies that a transition metal is involved in the C-H cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate. The intermediate of this first step can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".

Zethrene (dibenzo[de,mn]naphthacene) is a polycyclic aromatic hydrocarbon consisting of two phenalene units fused together. According to Clar's rule, the two exterior naphthalene units are truly aromatic and the two central double bonds are not aromatic at all. For this reason the compound is of some interest to academic research. Zethrene has a deep-red color and it is light sensitive - complete decomposition under a sunlight lamp occurs within 12 hours. The melting point is 262 °C.

Supermicelle is a hierarchical micelle structure where individual components are also micelles. Supermicelles are formed via bottom-up chemical approaches, such as self-assembly of long cylindrical micelles into radial cross-, star- or dandelion-like patterns in a specially selected solvent; solid nanoparticles may be added to the solution to act as nucleation centers and form the central core of the supermicelle. The stems of the primary cylindrical micelles are composed of various block copolymers connected by strong covalent bonds; within the supermicelle structure they are loosely held together by hydrogen bonds, electrostatic or solvophobic interactions.