
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.
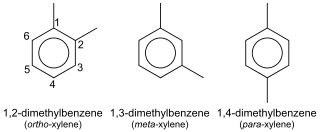
In organic chemistry, xylene or xylol are any of three organic compounds with the formula (CH3)2C6H4. They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value.
Isobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is n-heptane). It is an important component of gasoline, frequently used in relatively large proportions (around 10%) to increase the knock resistance of fuel.
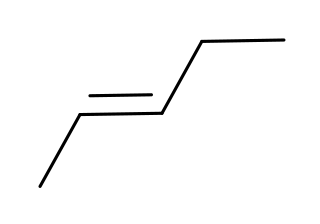
Pentenes are alkenes with the chemical formula C
5H
10. Each molecule contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure and whether the double bond has a cis or trans form.
p-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to monocyclic monoterpenes. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. p-Cymene is insoluble in water, but miscible with organic solvents.
Chlorotoluenes are aryl chlorides based on toluene in which at least one aromatic hydrogen atom is replaced with a chlorine atom. They have the general formula C7H8–nCln, where n = 1–5 is the number of chlorine atoms.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.

Prehnitene or 1,2,3,4-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Prehnitene is one of three isomers of tetramethylbenzene, the other two being isodurene (1,2,3,5-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene). It is a relatively easily oxidized benzene derivative, with E1/2 of 2.0 V vs NHE.
Pentamethylbenzene is an organic compound with the formula C6H(CH3)5. It is a colourless solid with a sweet odor. The compound is classified as an aromatic hydrocarbon. It is a relatively easily oxidized benzene derivative, with E1/2 of 1.95 V vs NHE.

An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains. Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups. The simplest member, toluene, has the hydrogen atom of the benzene ring replaced by a methyl group. The chemical formula of alkylbenzenes is CnH2n-6.
Cymene describes organic compounds with the formula CH3C6H4CH(CH3)2. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons. The bearing two substituents: an isopropyl group and a methyl group.
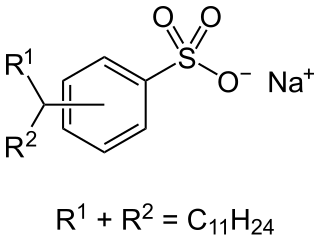
Alkylbenzene sulfonates are a class of anionic surfactants, consisting of a hydrophilic sulfonate head-group and a hydrophobic alkylbenzene tail-group. Along with sodium laureth sulfate, they are one of the oldest and most widely used synthetic detergents and may be found in numerous personal-care products and household-care products . They were introduced in the 1930s in the form of branched alkylbenzene sulfonates (BAS). However following environmental concerns these were replaced with linear alkylbenzene sulfonates (LAS) during the 1960s. Since then production has increased significantly from about one million tons in 1980, to around 3.5 million tons in 2016, making them most produced anionic surfactant after soaps.

Diethylbenzene (DEB) refers to any of three isomers with the formula C6H4(C2H5)2. Each consists of a benzene ring and two ethyl substituents. The meta and para have the greater commercial significance. All are colorless liquids.

4-Ethyltoluene is an organic compound with the formula CH3C6H4C2H5. It is one of three isomers of ethyltoluene, the other two isomers being 3-ethyltoluene and 2-ethyltoluene. All are colorless liquids and all are used for the production of specialty polystyrenes.
Ethyltolune describes organic compounds with the formula CH3C6H4CH2CH3. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons. The ring bears two substituents: a methyl group and an ethyl group.
The diisopropylbenzenes(DIPB) are organic compounds with the formula C6H4(CH(CH3)2)2. Three isomers exist: 1,2- 1,3-, and 1,4-diisopropylbenzene. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons bearing a pair of isopropyl (CH(CH3)2) substituents. DIPB has been referred to as "a common diluent" alongside hexane.

m-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring meta-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.













