The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base. The organoboron species is usually synthesized by hydroboration or carboboration, allowing for rapid generation of molecular complexity.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
In organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Such reactions often require the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction.
In chemistry, a transition metal pincer complex is a type of coordination complex with a pincer ligand. Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration. The inflexibility of the pincer-metal interaction confers high thermal stability to the resulting complexes. This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end. In the absence of this effect, cyclometallation is often a significant deactivation process for complexes, in particular limiting their ability to effect C-H bond activation. The organic substituents also define a hydrophobic pocket around the reactive coordination site. Stoichiometric and catalytic applications of pincer complexes have been studied at an accelerating pace since the mid-1970s. Most pincer ligands contain phosphines. Reactions of metal-pincer complexes are localized at three sites perpendicular to the plane of the pincer ligand, although in some cases one arm is hemi-labile and an additional coordination site is generated transiently. Early examples of pincer ligands were anionic with a carbanion as the central donor site and flanking phosphine donors; these compounds are referred to as PCP pincers.
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds. This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products.
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann.
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated carbon act as a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.

A Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
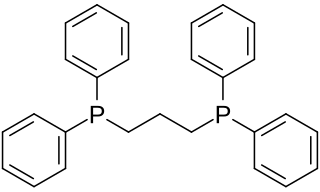
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this:
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Dichloro[1,3-bis(diphenylphosphino)propane]nickel a coordination complex with the formula NiCl2(dppp); where dppp is the diphosphine 1,3-bis(diphenylphosphino)propane. It is used as a catalyst in organic synthesis. The compound is a bright orange-red crystalline powder.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).
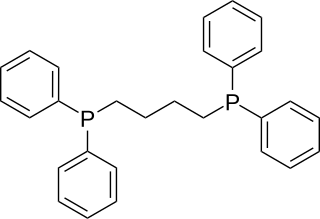
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.
Miyaura borylation, also known as the Miyaura borylation reaction, is a named reaction in organic chemistry that allows for the generation of boronates from vinyl or aryl halides with the cross-coupling of bis(pinacolato)diboron in basic conditions with a catalyst such as PdCl2(dppf). The resulting borylated products can be used as coupling partners for the Suzuki reaction.

trans,trans,trans-(1,5,9-Cyclododecatriene)nickel(0) a organonickel compound with the formula NiC12H18, better known as t-Ni(cdt). It is a 16-electron coordination complex featuring trigonal planar nickel(0) bound to the three alkene groups in the cyclododecatriene ligand. X-ray structural analysis demonstrates that the three olefins adopt a propeller-like arrangement around the nickel atom center, making the structure chiral. This extremely air-sensitive deep red solid was the first discovered Ni(0)-olefin complex.











