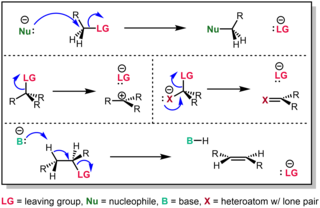
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term nucleofuge. In this context, leaving groups are generally anions or neutral species, departing from neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known.

The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds and are common ways of introducing functional groups into benzene rings. Some aliphatic compounds can undergo electrophilic substitution as well. H
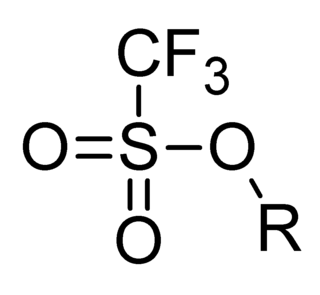
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.

Scandium(III) oxide or scandia is a inorganic compound with formula Sc2O3. It is one of several oxides of rare earth elements with a high melting point. It is used in the preparation of other scandium compounds as well as in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material).
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
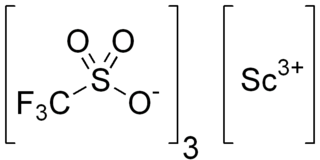
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO
3CF−
3 anions.

Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals, primarily phenol and acetone.
The Blanc chloromethylation is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923
Lanthanide triflates are triflate salts of the lanthanides. These salts have been investigated for application in organic synthesis as Lewis acid catalysts. These catalysts function similarly to aluminium chloride or ferric chloride, but they are water-tolerant (stable in water). Commonly written as Ln(OTf)3·(H2O)9 the nine waters are bound to the lanthanide, and the triflates are counteranions, so more accurately lanthanide triflate nonahydrate is written as [Ln(H2O)9](OTf)3.

Tetrachloroaluminate [AlCl4]− is an anion formed from aluminium and chlorine. The anion has a tetrahedral shape, similar to carbon tetrachloride where carbon is replaced with aluminium. Some tetrachloroaluminates are soluble in organic solvents, creating an ionic non-aqueous solution, making them suitable as component of electrolytes for batteries. For example, lithium tetrachloroaluminate is used in some lithium batteries.
The Minisci reaction is a named reaction in organic chemistry. It is a nucleophilic radical substitution to an electron deficient aromatic compound, most commonly the introduction of an alkyl group to a nitrogen containing heterocycle. The reaction was published in 1971 by F. Minisci. In the case of N-Heterocycles, the conditions must be acidic to ensure protonation of said heterocycle. A typical reaction is that between pyridine and pivalic acid with silver nitrate, sulfuric acid and ammonium persulfate to form 2-tert-butylpyridine. The reaction resembles Friedel-Crafts alkylation but with opposite reactivity and selectivity.
In organic chemistry, transalkylation is a chemical reaction involving the transfer of an alkyl group from one organic compound to another. The reaction is used for the transfer of methyl and ethyl groups between benzene rings. This is of particular value in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced. Zeolites are often used as catalysts in transalkylation reactions.
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, and alkylation and acylation Friedel–Crafts reaction.

1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.
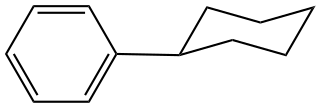
Cyclohexylbenzene is the organic compound with the structural formula C6H5−C6H11. It is a derivative of benzene with a cyclohexyl substituent (C6H11). It is a colorless liquid.

Hafnium(IV) triflate or hafnium trifluoromethansulfonate is an inorganic substance with the idealized formula Hf(OSO2CF3)4, also written as Hf(OTf)4. Hafnium triflate is used as an impure mixture as a catalyst. Hafnium (IV) has an ionic radius of intermediate range (Al < Ti < Hf < Zr < Sc < Ln) and has an oxophilic hard character typical of group IV metals. This solid is a stronger Lewis acid than its typical precursor hafnium tetrachloride, HfCl4, because of the strong electron-withdrawing nature of the four triflate groups, which makes it a great Lewis acid and has many uses including as a great catalyst at low Lewis acid loadings for electrophilic aromatic substitution and nucleophilic substitution reactions.












