
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is tert-butyl hydroperoxide. The method relies on a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate.
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal. It was discovered by the carbohydrate chemist Robert J. Ferrier.

2,3-Bisphosphoglyceric acid (2,3-BPG), also known as 2,3-diphosphoglyceric acid (2,3-DPG), is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid (1,3-BPG).

Bisphosphoglycerate mutase is an enzyme expressed in erythrocytes and placental cells. It is responsible for the catalytic synthesis of 2,3-Bisphosphoglycerate (2,3-BPG) from 1,3-bisphosphoglycerate. BPGM also has a mutase and a phosphatase function, but these are much less active, in contrast to its glycolytic cousin, phosphoglycerate mutase (PGM), which favors these two functions, but can also catalyze the synthesis of 2,3-BPG to a lesser extent.

Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis - the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM). The dPGM enzyme is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria. This class of PGM enzyme shares the same superfamily as alkaline phosphatase.

Sialyltransferases are enzymes that transfer sialic acid to nascent oligosaccharide. Each sialyltransferase is specific for a particular sugar substrate. Sialyltransferases add sialic acid to the terminal portions of the sialylated glycolipids (gangliosides) or to the N- or O-linked sugar chains of glycoproteins.

Indoleamine-pyrrole 2,3-dioxygenase (IDO or INDO EC 1.13.11.52) is a heme-containing enzyme physiologically expressed in a number of tissues and cells, such as the small intestine, lungs, female genital tract or placenta. In humans is encoded by the IDO1 gene. IDO is involved in tryptophan metabolism. It is one of three enzymes that catalyze the first and rate-limiting step in the kynurenine pathway, the O2-dependent oxidation of L-tryptophan to N-formylkynurenine, the others being indolamine-2,3-dioxygenase 2 (IDO2) and tryptophan 2,3-dioxygenase (TDO). IDO is an important part of the immune system and plays a part in natural defense against various pathogens. It is produced by the cells in response to inflammation and has an immunosuppressive function because of its ability to limit T-cell function and engage mechanisms of immune tolerance. Emerging evidence suggests that IDO becomes activated during tumor development, helping malignant cells escape eradication by the immune system. Expression of IDO has been described in a number of types of cancer, such as acute myeloid leukemia, ovarian cancer or colorectal cancer. IDO is part of the malignant transformation process and plays a key role in suppressing the anti-tumor immune response in the body, so inhibiting it could increase the effect of chemotherapy as well as other immunotherapeutic protocols. Furthermore, there is data implicating a role for IDO1 in the modulation of vascular tone in conditions of inflammation via a novel pathway involving singlet oxygen.

Lanosterol synthase (EC 5.4.99.7) is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.
The molecular formula C6H10 (molar mass: 82.14 g/mol) may refer to:
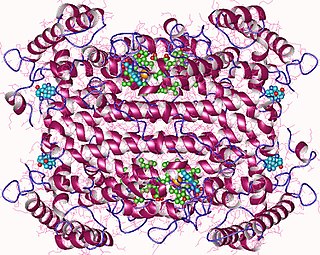
In enzymology, tryptophan 2,3-dioxygenase (EC 1.13.11.11) is a heme enzyme that catalyzes the oxidation of L-tryptophan (L-Trp) to N-formyl-L-kynurenine, as the first and rate-limiting step of the kynurenine pathway.
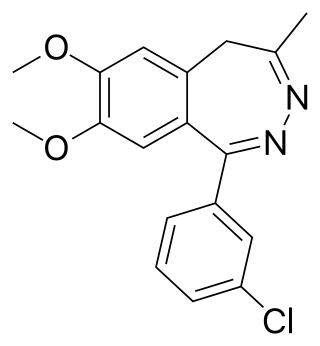
Girisopam is a drug which is a 2,3-benzodiazepine derivative, related to tofisopam and zometapine. It has selective anxiolytic action with no sedative, anticonvulsant or muscle relaxant effects.
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus and in the aquatic fern Salvinia molesta. It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound.

GYKI 52466 is a 2,3-benzodiazepine that acts as an ionotropic glutamate receptor antagonist, which is a non-competitive AMPA receptor antagonist (IC50 values are 10-20, ~ 450 and >> 50 μM for AMPA-, kainate- and NMDA-induced responses respectively), orally-active anticonvulsant, and skeletal muscle relaxant. Unlike conventional 1,4-benzodiazepines, GYKI 52466 and related 2,3-benzodiazepines do not act on GABAA receptors. Like other AMPA receptor antagonists, GYKI 52466 has anticonvulsant and neuroprotective properties.
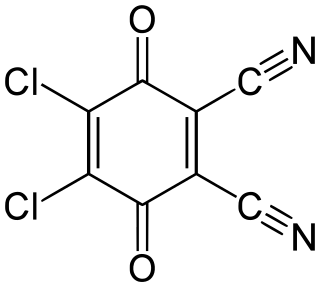
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid.
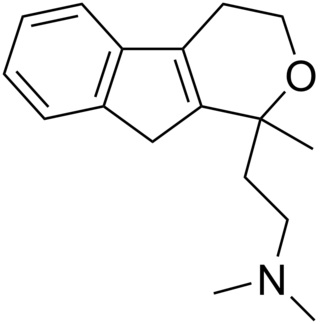
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.
The [2,3]-Wittig rearrangement is the transformation of an allylic ether into a homoallylic alcohol via a concerted, pericyclic process. Because the reaction is concerted, it exhibits a high degree of stereocontrol, and can be employed early in a synthetic route to establish stereochemistry. The Wittig rearrangement requires strongly basic conditions, however, as a carbanion intermediate is essential. [1,2]-Wittig rearrangement is a competitive process.

Epacadostat is an investigational drug for cancer. Epacadostat is an inhibitor of indoleamine 2,3-dioxygenase-1 (IDO1). Epacadostat inhibits IDO1 by competitively blocking it, without interfering with IDO2 or tryptophan 2,3-dioxygenase (TDO). It has antitumor activity in some models, though is most effective when combined with other immunotherapy agents.
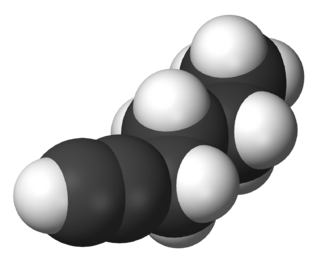
1-Hexyne is a hydrocarbon consisting of a straight six-carbon chain having a terminal alkyne. Its molecular formula is HC2C4H9. A colorless liquid, it is one of three isomers of hexyne. It is used as a reagent in organic synthesis.
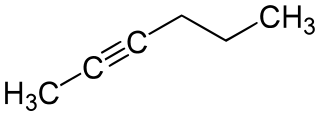
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.





















