In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon. This chemical reaction is useful in the organic synthesis of organic compounds.
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base. The reaction product is a 1,3-diyne or di-alkyne.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.

Disilyne is a silicon hydride with the formula Si
2H
2. Several isomers are possible, but none are sufficiently stable to be of practical value. Substituted disilynes contain a formal silicon–silicon triple bond and as such are sometimes written R2Si2 (where R is a substituent group). They are the silicon analogues of alkynes.

2-Pentyne, an organic compound with the formula CH3CH2C≡CCH3 and is an internal alkyne. It is an isomer of 1-pentyne, a terminal alkyne.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.
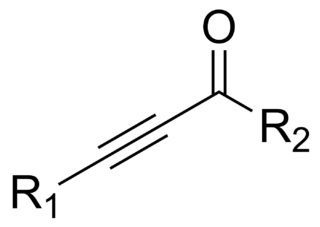
In organic chemistry, an ynone is an organic compound containing a ketone functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.
The Crabbé reaction is an organic reaction that converts a terminal alkyne and aldehyde into an allene in the presence of a soft Lewis acid catalyst and secondary amine. Given continued developments in scope and generality, it is a convenient and increasingly important method for the preparation of allenes, a class of compounds often viewed as exotic and synthetically challenging to access.
Diphosphorus tetrafluoride is a gaseous compound of phosphorus and fluorine with formula P2F4. Two fluorine atoms are connected to each phosphorus atom, and there is a bond between the two phosphorus atoms. Phosphorus can be considered to have oxidation state +2, as indicated by the name phosphorus difluoride.